Korean J Physiol Pharmacol.
2012 Dec;16(6):437-446. 10.4196/kjpp.2012.16.6.437.
Mechanisms of Motility Change on Trinitrobenzenesulfonic Acid-Induced Colonic Inflammation in Mice
- Affiliations
-
- 1Department of Gastroenterology, Gangneung Asan Medical Center, Gangneung 210-701, Korea.
- 2Department of Physiology, College of Medicine, Kwandong University, Gangneung 210-701, Korea. bgpark@kd.ac.kr
- KMID: 2285453
- DOI: http://doi.org/10.4196/kjpp.2012.16.6.437
Abstract
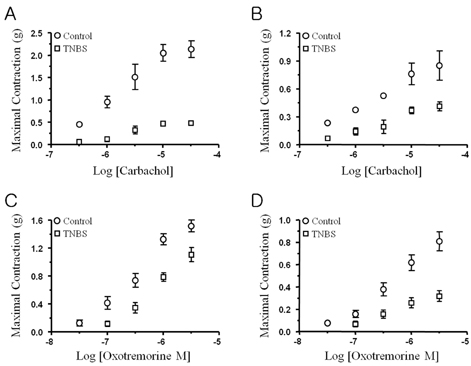
- Ulcerative colitis is an inflammatory bowel disease (IBD) characterized by recurrent episodes of colonic inflammation and tissue degeneration in human or animal models. The contractile force generated by the smooth muscle is significantly attenuated, resulting in altered motility leading to diarrhea or constipation in IBD. The aim of this study is to clarify the altered contractility of circular and longitudinal smooth muscle layers in proximal colon of trinitrobenzen sulfonic acid (TNBS)-induced colitis mouse. Colitis was induced by direct injection of TNBS (120 mg/kg, 50% ethanol) in proximal colon of ICR mouse using a 30 G needle anesthetized with ketamin (50 mg/kg), whereas animals in the control group were injected of 50% ethanol alone. In TNBS-induced colitis, the wall of the proximal colon is diffusely thickened with loss of haustration, and showed mucosal and mucular edema with inflammatory infiltration. The colonic inflammation is significantly induced the reduction of colonic contractile activity including spontaneous contractile activity, depolarization-induced contractility, and muscarinic acetylcholine receptor-mediated contractile response in circular muscle layer compared to the longitudinal muscle layer. The inward rectification of currents, especially, important to Ca2+ and Na+ influx-induced depolarization and contraction, was markedly reduced in the TNBS-induced colitis compared to the control. The muscarinic acetylcholine-mediated contractile responses were significantly attenuated in the circular and longitudinal smooth muscle strips induced by the reduction of membrane expression of canonical transient receptor potential (TRPC) channel isoforms from the proximal colon of the TNBS-induced colitis mouse than the control.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
A Review on Chemical-Induced Inflammatory Bowel Disease Models in Rodents
Puneet Kaur Randhawa, Kavinder Singh, Nirmal Singh, Amteshwar Singh Jaggi
Korean J Physiol Pharmacol. 2014;18(4):279-288. doi: 10.4196/kjpp.2014.18.4.279.
Reference
-
1. Reddy SN, Bazzocchi G, Chan S, Akashi K, Villanueva-Meyer J, Yanni G, Mena I, Snape WJ Jr. Colonic motility and transit in health and ulcerative colitis. Gastroenterology. 1991. 101:1289–1297.2. Annese V, Bassotti G, Napolitano G, Usai P, Andriulli A, Vantrappen G. Gastrointestinal motility disorders in patients with inactive Crohn's disease. Scand J Gastroenterol. 1997. 32:1107–1117.3. Martinolle JP, Garcia-Villar R, Fioramonti J, Bueno L. Altered contractility of circular and longitudinal muscle in TNBS-inflamed guinea pig ileum. Am J Physiol. 1997. 272:G1258–G1267.4. Jacobson K, McHugh K, Collins SM. Experimental colitis alters myenteric nerve function at inflamed and noninflamed sites in the rat. Gastroenterology. 1995. 109:718–722.5. Lin A, Lourenssen S, Stanzel RD, Blennerhassett MG. Selective loss of NGF-sensitive neurons following experimental colitis. Exp Neurol. 2005. 191:337–343.6. Lu G, Qian X, Berezin I, Telford GL, Huizinga JD, Sarna SK. Inflammation modulates in vitro colonic myoelectric and contractile activity and interstitial cells of Cajal. Am J Physiol. 1997. 273:G1233–G1245.7. Chang IY, Glasgow NJ, Takayama I, Horiguchi K, Sanders KM, Ward SM. Loss of interstitial cells of Cajal and development of electrical dysfunction in murine small bowel obstruction. J Physiol. 2001. 536:555–568.8. Blennerhassett MG, Vignjevic P, Vermillion DL, Collins SM. Inflammation causes hyperplasia and hypertrophy in smooth muscle of rat small intestine. Am J Physiol. 1992. 262:G1041–G1046.9. Malykhina AP, Akbarali HI. Inflammation-induced "channelopathies" in the gastrointestinal smooth muscle. Cell Biochem Biophys. 2004. 41:319–330.10. Akbarali HI, Pothoulakis C, Castagliuolo I. Altered ion channel activity in murine colonic smooth muscle myocytes in an experimental colitis model. Biochem Biophys Res Commun. 2000. 275:637–642.11. Shi XZ, Sarna SK. Impairment of Ca2+ mobilization in circular muscle cells of the inflamed colon. Am J Physiol Gastrointest Liver Physiol. 2000. 278:G234–G242.12. Kinoshita K, Sato K, Hori M, Ozaki H, Karaki H. Decrease in activity of smooth muscle L-type Ca2+ channels and its reversal by NF-kappaB inhibitors in Crohn's colitis model. Am J Physiol Gastrointest Liver Physiol. 2003. 285:G483–G493.13. Kang M, Morsy N, Jin X, Lupu F, Akbarali HI. Protein and gene expression of Ca2+ channel isoforms in murine colon: effect of inflammation. Pflugers Arch. 2004. 449:288–297.14. Jin X, Malykhina AP, Lupu F, Akbarali HI. Altered gene expression and increased bursting activity of colonic smooth muscle ATP-sensitive K+ channels in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2004. 287:G274–G285.15. Sawyer GW, Ehlert FJ. Muscarinic M3 receptor inactivation reveals a pertussis toxin-sensitive contractile response in the guinea pig colon: evidence for M2/M3 receptor interactions. J Pharmacol Exp Ther. 1999. 289:464–476.16. Shi XZ, Sarna SK. Inflammatory modulation of muscarinic receptor activation in canine ileal circular muscle cells. Gastroenterology. 1997. 112:864–874.17. Ehlert FJ, Sawyer GW, Esqueda EE. Contractile role of M2 and M3 muscarinic receptors in gastrointestinal smooth muscle. Life Sci. 1999. 64:387–394.18. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981. 391:85–100.19. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001. 25:402–408.20. Snape WJ Jr, Matarazzo SA, Cohen S. Abnormal gastrocolonic response in patients with ulcerative colitis. Gut. 1980. 21:392–396.21. Vermillion DL, Huizinga JD, Riddell RH, Collins SM. Altered small intestinal smooth muscle function in Crohn's disease. Gastroenterology. 1993. 104:1692–1699.22. Hibi T, Ogata H, Sakuraba A. Animal models of inflammatory bowel disease. J Gastroenterol. 2002. 37:409–417.23. Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989. 96:795–803.24. Boirivant M, Fuss IJ, Chu A, Strober W. Oxazolone colitis: A murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J Exp Med. 1998. 188:1929–1939.25. Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990. 98:694–702.26. Marcus AJ, Marcus SN, Marcus R, Watt J. Rapid production of ulcerative disease of the colon in newly-weaned guinea-pigs by degraded carrageenan. J Pharm Pharmacol. 1989. 41:423–426.27. Kitsukawa Y, Saito H, Suzuki Y, Kasanuki J, Tamura Y, Yoshida S. Effect of ingestion of eicosapentaenoic acid ethyl ester on carrageenan-induced colitis in guinea pigs. Gastroenterology. 1992. 102:1859–1866.28. Jurjus AR, Khoury NN, Reimund JM. Animal models of inflammatory bowel disease. J Pharmacol Toxicol Methods. 2004. 50:81–92.29. Yamada Y, Marshall S, Specian RD, Grisham MB. A comparative analysis of two models of colitis in rats. Gastroenterology. 1992. 102:1524–1534.30. Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995. 109:1344–1367.31. Depoortere I, Van Assche G, Thijs T, Geboes K, Peeters TL. Differential changes in ACh-, motilin-, substance P-, and K+-induced contractility in rabbit colitis. Am J Physiol. 1999. 277:G61–G68.32. Kwon SC, Won KJ, Jung SH, Lee KP, Lee DY, Park ES, Kim B, Cheon GJ, Han KH. Proteomic analysis of colonic mucosal tissue from tuberculous and ulcerative colitis patients. Korean J Physiol Pharmacol. 2012. 16:193–198.33. Sarna SK. Neuronal locus and cellular signaling for stimulation of ileal giant migrating and phasic contractions. Am J Physiol Gastrointest Liver Physiol. 2003. 284:G789–G797.34. Kinoshita K, Horiguchi K, Fujisawa M, Kobirumaki F, Yamato S, Hori M, Ozaki H. Possible involvement of muscularis resident macrophages in impairment of interstitial cells of Cajal and myenteric nerve systems in rat models of TNBS-induced colitis. Histochem Cell Biol. 2007. 127:41–53.35. Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996. 111:492–515.36. Thuneberg L. Interstitial cells of Cajal: intestinal pacemaker cells? Adv Anat Embryol Cell Biol. 1982. 71:1–130.37. Faussone-Pellegrini MS, Thuneberg L. Guide to the identification of interstitial cells of Cajal. Microsc Res Tech. 1999. 47:248–266.38. Rumessen JJ. Identification of interstitial cells of Cajal. Significance for studies of human small intestine and colon. Dan Med Bull. 1994. 41:275–293.39. Porcher C, Baldo M, Henry M, Orsoni P, Julé Y, Ward SM. Deficiency of interstitial cells of Cajal in the small intestine of patients with Crohn's disease. Am J Gastroenterol. 2002. 97:118–125.40. Liu X, Rusch NJ, Striessnig J, Sarna SK. Down-regulation of L-type calcium channels in inflamed circular smooth muscle cells of the canine colon. Gastroenterology. 2001. 120:480–489.41. Kinoshita K, Sato K, Hori M, Ozaki H, Karaki H. Decrease in activity of smooth muscle L-type Ca2+ channels and its reversal by NF-kappaB inhibitors in Crohn's colitis model. Am J Physiol Gastrointest Liver Physiol. 2003. 285:G483–G493.42. Ohama T, Hori M, Ozaki H. Mechanism of abnormal intestinal motility in inflammatory bowel disease: how smooth muscle contraction is reduced? J Smooth Muscle Res. 2007. 43:43–54.43. Shi XZ, Pazdrak K, Saada N, Dai B, Palade P, Sarna SK. Negative transcriptional regulation of human colonic smooth muscle Cav1.2 channels by p50 and p65 subunits of nuclear factor-kappaB. Gastroenterology. 2005. 129:1518–1532.44. Wegener JW, Schulla V, Koller A, Klugbauer N, Feil R, Hofmann F. Control of intestinal motility by the Ca(v)1.2 L-type calcium channel in mice. FASEB J. 2006. 20:1260–1262.45. Stengel PW, Gomeza J, Wess J, Cohen ML. M2 and M4 receptor knockout mice: muscarinic receptor function in cardiac and smooth muscle in vitro. J Pharmacol Exp Ther. 2000. 292:877–885.46. Matsui M, Motomura D, Fujikawa T, Jiang J, Takahashi S, Manabe T, Taketo MM. Mice lacking M2 and M3 muscarinic acetylcholine receptors are devoid of cholinergic smooth muscle contractions but still viable. J Neurosci. 2002. 22:10627–10632.47. Preiksaitis HG, Krysiak PS, Chrones T, Rajgopal V, Laurier LG. Pharmacological and molecular characterization of muscarinic receptor subtypes in human esophageal smooth muscle. J Pharmacol Exp Ther. 2000. 295:879–888.48. Jin X, Morsy N, Shoeb F, Zavzavadjian J, Akbarali HI. Coupling of M2 muscarinic receptor to L-type Ca channel via c-src kinase in rabbit colonic circular smooth muscle. Gastroenterology. 2002. 123:827–834.49. Shi XZ, Sarna SK. Differential inflammatory modulation of canine ileal longitudinal and circular muscle cells. Am J Physiol. 1999. 277:G341–G350.50. Jadcherla SR. Inflammation inhibits muscarinic signaling in in vivo canine colonic circular smooth muscle cells. Pediatr Res. 2002. 52:756–762.51. Bolton TB. Cholinergic mechanisms in smooth muscle. Br Med Bull. 1979. 35:275–283.52. Benham CD, Bolton TB, Lang RJ. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985. 316:345–347.53. Inoue R, Isenberg G. Acetylcholine activates nonselective cation channels in guinea pig ileum through a G protein. Am J Physiol. 1990. 258:C1173–C1178.54. Zhu MH, Lee YM, Jin N, So I, Kim KW. The transient receptor potential protein homologue TRPC4/5 as a candidate for the nonselective cationic channel activated by muscarinic stimulation in the murine stomach. Neurophysiol. 2003. 35:302–307.55. Venkatachalam K, van Rossum DB, Patterson RL, Ma HT, Gill DL. The cellular and molecular basis of store-operated calcium entry. Nat Cell Biol. 2002. 4:E263–E272.56. Freichel M, Vennekens R, Olausson J, Hoffmann M, Müller C, Stolz S, Scheunemann J, Weissgerber P, Flockerzi V. Functional role of TRPC proteins in vivo: lessons from TRPC-deficient mouse models. Biochem Biophys Res Commun. 2004. 322:1352–1358.57. Clapham DE. TRP channels as cellular sensors. Nature. 2003. 426:517–524.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Saccharomyces boulardii Reduced Intestinal Inflammation in Mice Model of 2,4,6-trinitrobencene Sulfonic Acid Induced Colitis: Based on Microarray
- Altered Colonic Motor Functions in Experimental Colitis of Guinea Pigs
- Changes in Colonic Transit and Contractility of Muscle According to The Time Course in TNBS-Induced Colitis
- Altered Colonic Transit in TNBS-induced Experimental Colitis in Guinea Pig and Distribution of Nitric Oxide Synthase in the Colonic Wall
- Motility Disorders of the Colon