Korean J Physiol Pharmacol.
2009 Feb;13(1):39-47. 10.4196/kjpp.2009.13.1.39.
Mechanisms of Selective Antimicrobial Activity of Gaegurin 4
- Affiliations
-
- 1Laboratories of Veterinary Pharmacology and Biochemistry, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. pdryu@snu.ac.kr
- 2Laboratory of Molecular Genetics, Institute for Molecular Biology and Genetics and Department of Microbiology, Seoul National University, Seoul 151-742, Korea.
- 3Department of Physiology, Institute of Health Sciences and Medical Research Center for Neural Dysfunction, College of Medicine, Gyeongsang National University, Jinju 660-751, Korea.
- KMID: 2285370
- DOI: http://doi.org/10.4196/kjpp.2009.13.1.39
Abstract
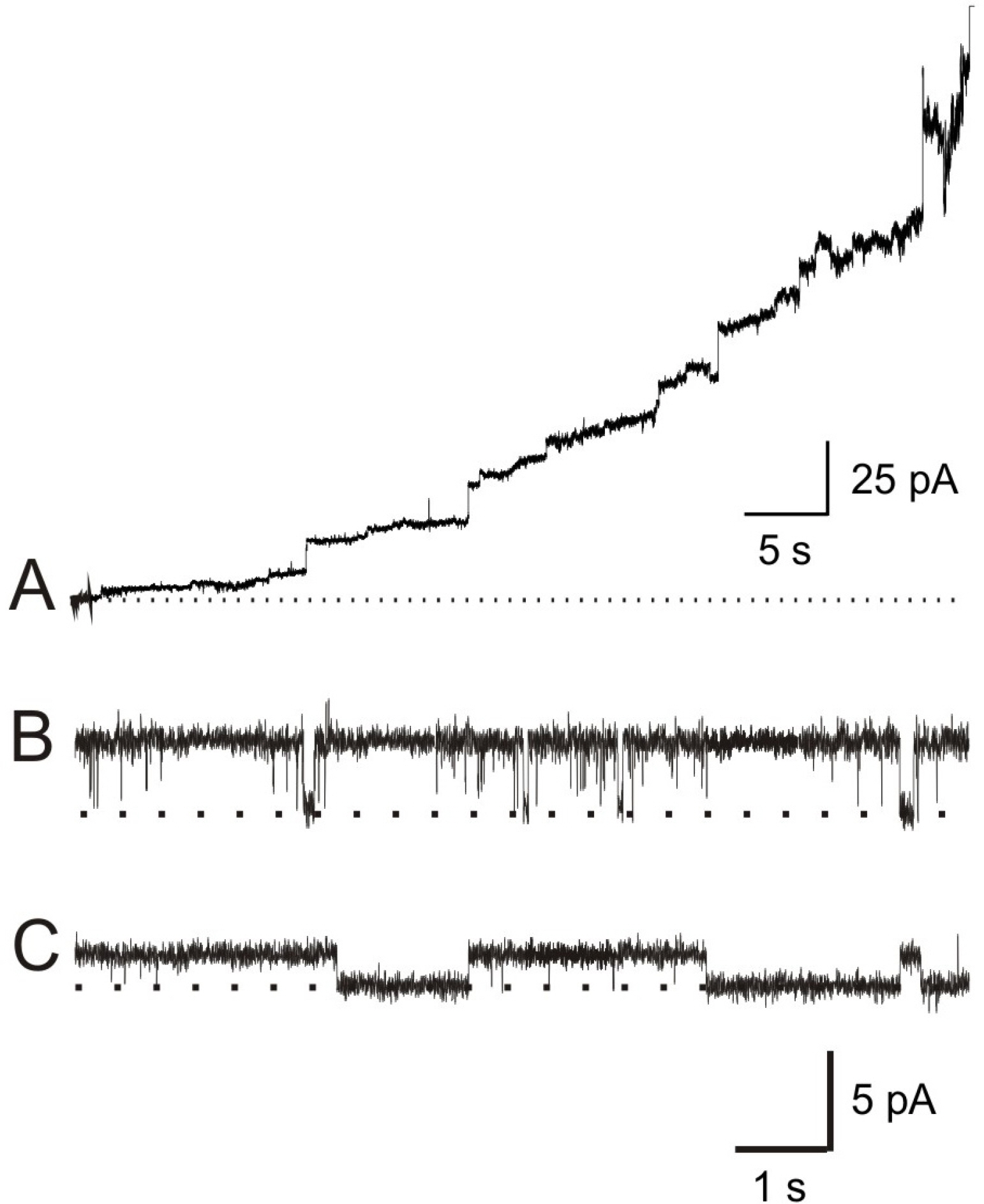
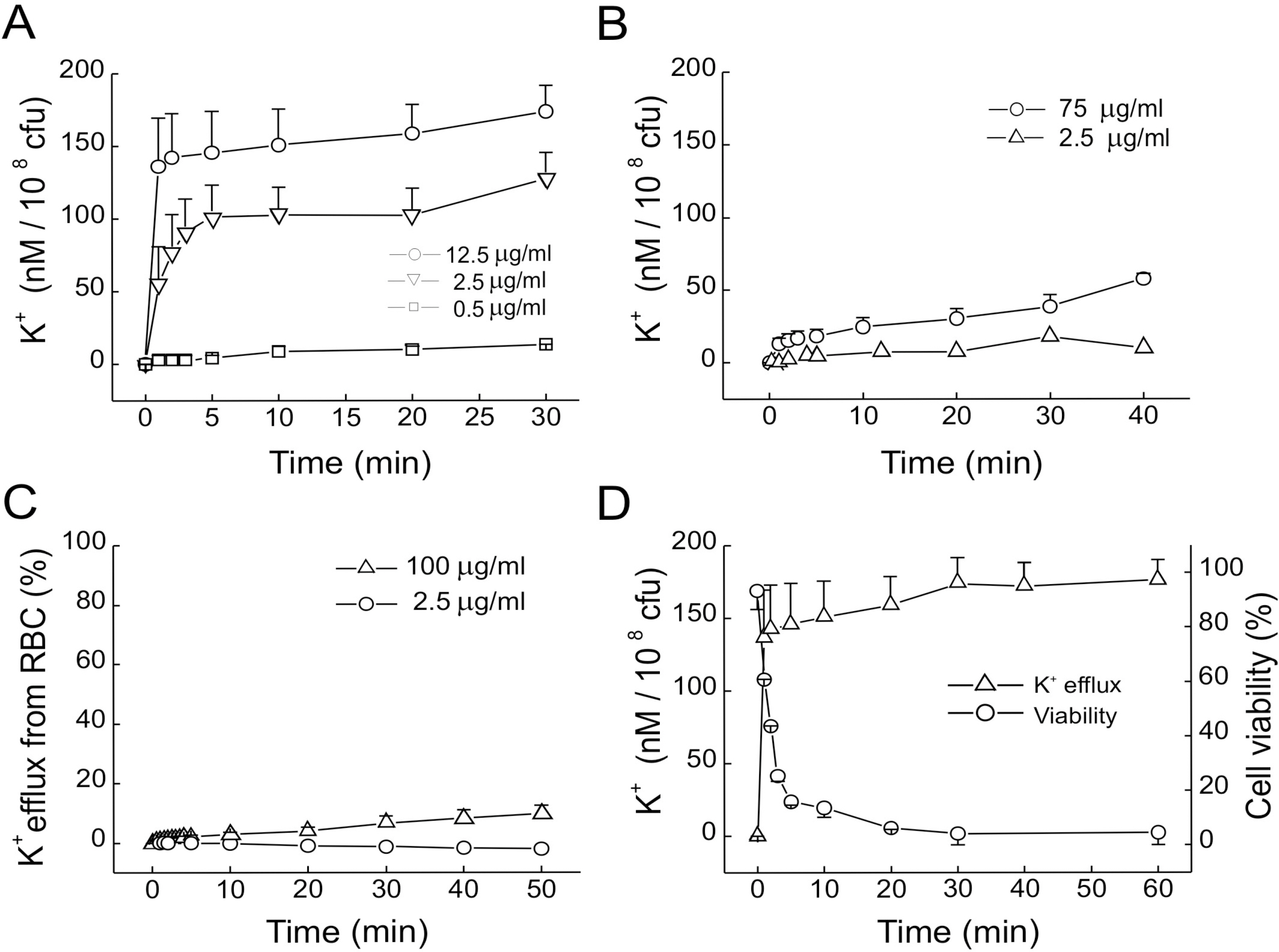
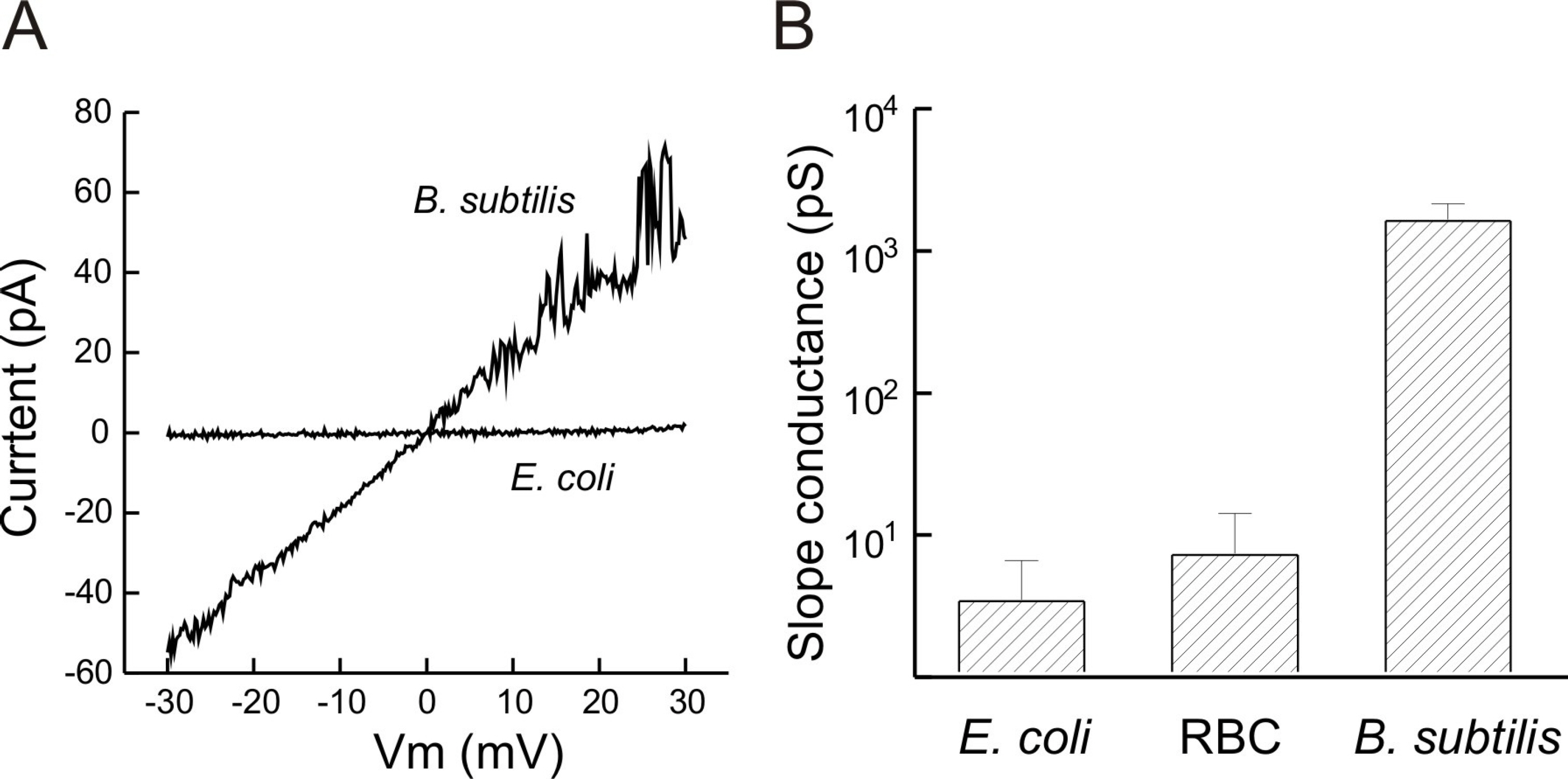
- Gaegurin 4 (GGN4), an antimicrobial peptide isolated from a Korean frog, is five times more potent against Gram-positive than Gram-negative bacteria, but has little hemolytic activity. To understand the mechanism of such cell selectivity, we examined GGN4-induced K+ efflux from target cells, and membrane conductances in planar lipid bilayers. The K+ efflux from Gram-positive M. luteus (2.5microgram/ml) was faster and larger than that from Gram-negative E. coli (75microgram/ml), while that from RBC was negligible even at higher concentration (100microgram/ml). GGN4 induced larger conductances in the planar bilayers which were formed with lipids extracted from Gram-positive B. subtilis than in those from E. coli (p<0.01), however, the effects of GGN4 were not selective in the bilayers formed with lipids from E. coli and red blood cells. Addition of an acidic phospholipid, phosphatidylserine to planar bilayers increased the GGN4-induced membrane conductance (p<0.05), but addition of phosphatidylcholine or cholesterol reduced it (p<0.05). Transmission electron microscopy revealed that GGN4 induced pore-like damages in M. luteus and dis-layering damages on the outer wall of E. coli. Taken together, the present results indicate that the selectivity of GGN4 toward Gram-positive over Gram-negative bacteria is due to negative surface charges, and interaction of GGN4 with outer walls. The selectivity toward bacteria over RBC is due to the presence of phosphatidylcholine and cholesterol, and the trans-bilayer lipid asymmetry in RBC. The results suggest that design of selective antimicrobial peptides should be based on the composition and topology of membrane lipids in the target cells.
MeSH Terms
Figure
Reference
-
Agre P., Bennett V. Qualitative and functional analyses of spectrin, ankyrin, band 3, and calmodulin in human red cell membranes. Methods Hematol. 19:95–98. 1988.Ames GF. Lipids of Salmonella tryphimurium and Escherichia coli: structure and metabolism. J Bacteriol. 95:833–843. 1968.Balasubramanian K., Schroit AJ. Aminophospholipid asymmetry: a matter of life and death. Annu Rev Physiol. 65:701–734. 2003.
ArticleBishop DG., Rutberg L., Samuelsson B. The chemical composition of the cytoplasmic membrane of Bacillus subtilis. Eur J Biochem. 2:448–453. 1967.
ArticleBishop EA., Bermingham MAC. Lipid composition of Gram-negative bacteria, sensitive and resistant to streptomycin. Antimicrobial Agents Chem. 4:378–379. 1973.
ArticleBloch K. Choleterol: evolution of structure and function. Vance DE, Vance J, editors. ed,. Biochemistry of lipids and membranes. Amsterdam: Elesevier Science Publishers;p. p. 363–382. 1991.Boman HG. Innate immunity and the normal microflora. Immunol Rev. 173:5–16. 2000.
ArticleChi SW., Kim JS., Kim DH., Lee SH., Park YH., Han KH. Solution structure and membrane interaction mode of an antimicrobial peptide gaegurin 4. Biochem Biophys Res Commun. 352:592–597. 2007.
ArticleClark DP., Durell S., Maloy WL., Zasloff M. Ranalexin: a novel antimicrobial peptide from bullfrog (Rana catesbeiana) skin, structurally related to bacterial antibiotic, polymyxin. J Biol Chem. 269:10849–10855. 1994.Clejan S., Krulwicj TA., Mondrus KR., Sept-Young D. Membrane lipid composition of obligately and facultatively alkalophilic strains of Bacillus spp. J Bacteriol. 168:334–340. 1986.
ArticleConlon JM. Reflections on a systematic nomenclature for antimicrobial peptides from the skins of frogs of the family Ranidae. Peptides. 29:1815–1819. 2008.
ArticleCronan JE., Roy-Vagelos P. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 165:379–387. 1972.
ArticleDaleke DL. Regulation of phospholipid asymmetry in the erythrocyte membrane. Curr Opin Hematol. 15:191–195. 2008.
ArticleDathe M., Schumann M., Wieprecht T., Winkler A., Beyermann M., Krause E., Matsuzaki K., Murase O., Bienert M. Peptide helicity and membrane surface charge modulate the balance of electrostatic and hydrophobic interactions with lipid bilayers and biological membranes. Biochemistry. 35:12612–12622. 1996.
ArticleDowhan W. Molecular basis for membrane phospholipid diversity: why are there so many lipids? Ann Rev Biochem. 66:199–232. 1997.
ArticleEun SY., Jang HK., Han SK., Ryu PD., Lee BJ., Han KH., Kim SJ. A helix-induced oligomeric transition of gaegurin 4, an antimicrobial peptide isolated from a Korean frog. Mol Cells. 21:229–236. 2006.Gennis RB. The structure and composition of biomembranes. Gennis RB, editor. ed,. Biomembranes, molecular structure and functions. New York: Springer Verlag;p. p. 1–35. 1991.Gidalevitz D., Ishitsuka Y., Muresan AS., Konovalov O., Waring AJ., Lehrer RI., Lee KY. Interaction of antimicrobial peptide protegrin with biomembranes. Proc Natl Acad Sci USA. 100:6302–6307. 2003.
ArticleKagan B., Selsted ME., Ganz T., Lehrer RI. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc Natl Acad Sci USA. 87:210–214. 1990.
ArticleKim HJ., Han SK., Park JB., Baek HJ., Lee BJ., Ryu PD. Gaegurin 4, a peptide antibiotic of frog skin, forms voltage-dependent channels in planar lipid bilayers. J Pept Res. 53:1–7. 1999.
ArticleKim HJ., Kim SS., Lee MH., Lee BJ., Ryu PD. Role of C-terminal heptapeptide in pore-forming activity of antimicrobial agent, gaegurin 4. J Pept Res. 64:151–158. 2004.
ArticleKim KS., Fulton RW. Ultrastructure of Datura stramonium infected with an euphorbia virus suggestive of witefly-transmitted germinivirus. Phytopathology. 74:236–241. 1984.Lau YH., Caswell AH., Brunschwig J., Baerwald RJ., Garcia M. Lipid analysis and freeze-fracture studies on isolated transverse tubules and sarcoplasmic reticulum subfractions of skeletal muscle. J Biol Chem. 254:540–546. 1979.
ArticleMatsumoto K., Kusaka J., Nishibori A., Hara H. Lipid domains in bacterial membranes. Mol Microbiol. 61:1110–1117. 2006.
ArticleMatsuzaki K. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim Biophys Acta. 1462:1–10. 1999.
ArticleMatsuzaki K. Control of cell selectivity of antimicrobial peptides. Biochim Biophys Acta. (In press).
ArticleMatsuzaki K., Harada M., Handa T., Funakoshi S., Fujii N., Yajima H., Miyajima K. Magainin 1-induced leakage of entrapped calcein out of negatively-charged lipid vesicles. Biochim Biophys Acta. 981:130–134. 1989.
ArticleMatsuzaki K., Sugishita K., Fujii N., Miyajima K. Molecular basis for membrane selectivity of an antimicrobial peptide, magainin 2. Biochemistry. 34:3423–3429. 1995.
ArticleMorein S., Andersson AS., Rilfors L., Lindblom G. Wild-type Escherichia coli cells regulate the membrane lipid composition in a “Window” between gel and non-lamellar structures. J Biol Chem. 271:6801–6809. 1996.
ArticleMorikawa N., Hagiwara K., Nakajima T. Brevinin-1 and brevinin-2, unique antimicrobial peptides from the skin of the frog Rana brevipoda porsa. Biochem Biophys Res Comm. 189:184–190. 1992.Orlov DS., Nguyen T., Lehrer RI. Potassium release, a useful tool for studying antimicrobial peptides. Microbiol Methods. 49:325–328. 2002.
ArticlePark JB., Kim HJ., Ryu PD., Moczydlowski E. Effect of phosphatidylserine on unitary conductance and Ba2+ block of the BK Ca2+-activated K+ channel: re-examination of the surface charge hypothesis. J Gen Physiol. 121:375–398. 2003.Park S., Son WS., Kim YJ., Kwon AR., Lee BJ. NMR spectroscopic assessment of the structure and dynamic properties of an amphibian antimicrobial peptide (Gaegurin 4) bound to SDS micelles. J Biochem Mol Biol. 40:261–269. 2007.
ArticlePark SH., Kim YK., Park JW., Lee BJ., Lee BJ. Solution structure of the antimicrobial peptide gaegurin 4 1H and 15N nuclear magnetic resonance spectroscopy. Eur J Biochem. 267:2695–2704. 2000.Park JM., Jung JE., Lee BJ. Antimicrobial peptides from the skin of a Korean frog, Rana rugosa. Biochem Biophys Res Comm. 205:948–954. 1994.
ArticleShai Y. From innate immunity to de-novo designed antimicrobial peptides. Curr Pharm Des. 8:715–725. 2002.
ArticleSimmaco M., Mignogna G., Barra D., Bossa F. Novel antimicrobial peptides from skin secretion of the European frog, Rana esculenta. FEBA Lett. 324:159–161. 1993.Won HS., Kang SJ., Lee BJ. Action mechanism and structural requirements of the antimicrobial peptides, gaegurins. Biochim Biophys Acta. 2009. (In press).
ArticleZasloff M. Antimicrobial peptides of multicellular organisms. Nature. 415:389–395. 2002.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antimicrobial activity of candidate probiotic Streptococcus salivarius against Gram-positive bacteria in oral cavity
- Interpretation of Antimicrobial Susceptibility Test According to Resistance Mechanism of beta-lactam in Enterobacteriacae
- Characteristics of antimicrobial activity of Streptococcus salivarius K12 by culture condition
- Antimicrobials and Antimicrobial Resistant Superbacteria
- Effectiveness of mentha extracts against oral microorganisms: an in vitro study