J Adv Prosthodont.
2013 May;5(2):110-117. 10.4047/jap.2013.5.2.110.
The effect of acrylamide incorporation on the thermal and physical properties of denture resins
- Affiliations
-
- 1Department of Prosthodontics, Faculty of Dentistry, Karadeniz Technical University, Trabzon, Turkey. aydelif@hotmail.com
- 2Department of Prosthodontics, Faculty of Dentistry, Afyon Kocatepe University, Afyon, Turkey.
- 3Department of Prosthodontics, Faculty of Dentistry, Katip Celebi University, Izmir, Turkey.
- KMID: 2284749
- DOI: http://doi.org/10.4047/jap.2013.5.2.110
Abstract
- PURPOSE
Polymethyl methacrylate (PMMA) is the most commonly used denture base material despite typically low in strength. The purpose of this study was to improve the physical properties of the PMMA based denture base resins (QC-20, Dentsply Ltd., Addlestone, UK; Stellon, AD International Ltd, Dentsply, Switzerland; Acron MC; GC Lab Technologies Inc., Alsip, Japan) by copolymerization mechanism.
MATERIALS AND METHODS
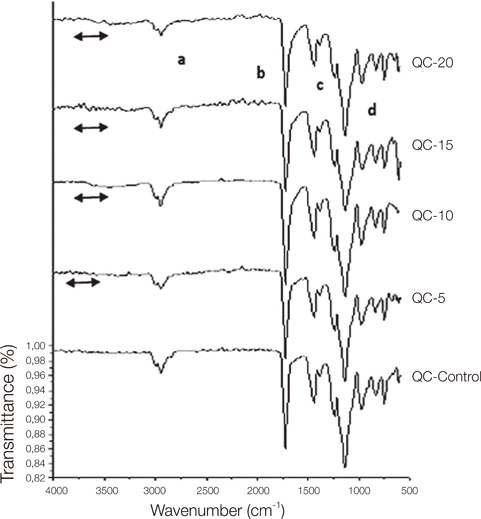
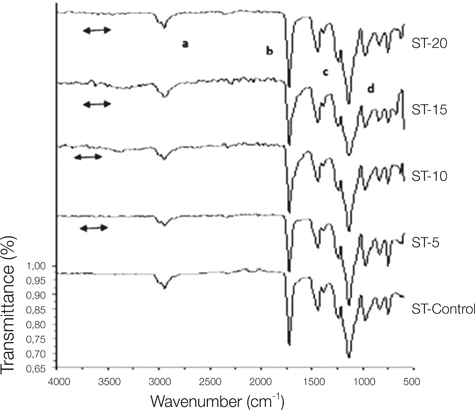
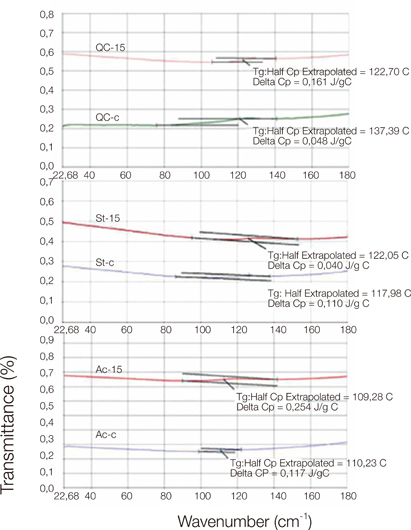
Control group specimens were prepared according to the manufacturer recommendations. In the copolymer groups; resins were prepared with 5%, 10%, 15% and 20% acrylamide (AAm) (Merck, Hohenbrunn, Germany) content according to the moleculer weight ratio, respectively. Chemical structure was characterized by a Bruker Vertex-70 Fourier transform infrared spectroscopy (FTIR) (Bruker Optics Inc., Ettlingen, Germany). Hardness was determined using an universal hardness tester (Struers Duramin, Struers A/S, Ballerup, Denmark) equipped with a Vickers diamond penetrator. The glass transition temperature (Tg) of control and copolymers were evaluated by Perkin Elmer Diamond DSC (Perkin Elmer, Massachusetts,USA). Statistical analyses were carried out using the statistical package SPSS for Windows, version 15.0 (SPSS, Chicago, IL, USA). The results were tested regarding the normality of distribution with the Shapiro Wilk test. Data were analyzed using ANOVA with post-hoc Tukey test (P<.01).
RESULTS
The copolymer synthesis was confirmed by FTIR spectroscopy. Glass transition temperature of the copolymer groups were higher than the control groups of the resins. The 10%, 15% and 20% copolymer groups of Stellon presented significantly higher than the control group in terms of hardness. 15% and 20% copolymer groups of Acron MC showed significantly higher hardness values when compared to the control group of the resin. Acrylamide addition did not affect the hardness of the QC-20 resin significantly.
CONCLUSION
Within the limitation of this study, it can be concluded that copolymerization of PMMA with AAm increased the hardness value and glass transition temperature of PMMA denture base resins.
Keyword
MeSH Terms
Figure
Reference
-
1. Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. The global burden of oral diseases and risks to oral health. Bull World Health Organ. 2005; 83:661–669.2. Tang AT, Li J, Ekstrand J, Liu Y. Cytotoxicity tests of in situ polymerized resins: methodological comparisons and introduction of a tissue culture insert as a testing device. J Biomed Mater Res. 1999; 45:214–222.3. Rached RN, Del-Bel Cury AA. Heat-cured acrylic resin repaired with microwave-cured one: bond strength and surface texture. J Oral Rehabil. 2001; 28:370–375.4. Wolfaardt JF, Cleaton-Jones P, Fatti P. The occurrence of porosity in a heat-cured poly (methyl methacrylate) denture base resin. J Prosthet Dent. 1986; 55:393–400.5. Honorez P, Catalan A, Angnes U, Grimonster J. The effect of three processing cycles on some physical and chemical properties of a heat-cured acrylic resin. J Prosthet Dent. 1989; 61:510–517.6. Nishii M. Curing of denture base resins with microwave irradiation: with particular reference to heat-curing resins. J Osaka Dent Univ. 1968; 2:23–40.7. Barbosa DB, Monteiro DR, Barão VA, Pero AC, Compagnoni MA. Effect of monomer treatment and polymerisation methods on the bond strength of resin teeth to denture base material. Gerodontology. 2009; 26:225–231.8. Jacob J, Chia LHL, Boey FYC. Comparative study of methyl methacrylate cure by microwave radiation versus thermal energy. Polym Test. 1995; 14:343–354.9. Smith LT, Powers JM, Ladd D. Mechanical properties of new denture resins polymerized by visible light, heat, and microwave energy. Int J Prosthodont. 1992; 5:315–320.10. De Clerck JP. Microwave polymerization of acrylic resins used in dental prostheses. J Prosthet Dent. 1987; 57:650–658.11. Jerolimov V, Brooks SC, Huggett R, Bates JF. Rapid curing of acrylic denture-base materials. Dent Mater. 1989; 5:18–22.12. Usanmaz A, Ateş J, Doğan A. Thermal and Mechanical Properties of Microwave- and Heat-Cured Poly(methyl methacrylate) Used as Dental Base Material. J Appl Polym Sci. 2003; 90:251–256.13. Bural C, Aktaş E, Deniz G, Ünlüçerçi Y, Bayraktar G. Effect of leaching residual methyl methacrylate concentrations on in vitro cytotoxicity of heat polymerized denture base acrylic resin processed with different polymerization cycles. J Appl Oral Sci. 2011; 19:306–312.14. Takahashi T, Gonda T, Maeda Y. Influence of reinforcing materials on strain of maxillary complete denture. Acta Odontol Scand. 2013; 71:307–311.15. Beyli MS, von Fraunhofer JA. An analysis of causes of fracture of acrylic resin dentures. J Prosthet Dent. 1981; 46:238–241.16. Jagger DC, Harrison A, Jandt KD. The reinforcement of dentures. J Oral Rehabil. 1999; 26:185–194.17. Cunha TR, Regis RR, Bonatti MR, de Souza RF. Influence of incorporation of fluoroalkyl methacrylates on roughness and flexural strength of a denture base acrylic resin. J Appl Oral Sci. 2009; 17:103–107.18. Moszner N, Fischer UK, Angermann J, Rheinberger V. Bis-(acrylamide)s as new cross-linkers for resin-based composite restoratives. Dent Mater. 2006; 22:1157–1162.19. Umemoto K, Kurata S. Basic study of a new denture base resin applying hydrophobic methacrylate monomer. Dent Mater J. 1997; 16:21–30.20. Umemoto K, Kurata S, Morishita K, Kawase T. Basic study of a new soft resin applied with bisfunctional siloxane oligomer. Dent Mater J. 2007; 26:656–658.21. Mortimer DA. Synthetic polyelectrolytes- A review. Polym Int. 1991; 25:29–41.22. Friedman M. Chemistry, biochemistry, and safety of acrylamide. A review. J Agric Food Chem. 2003; 51:4504–4526.23. Pinto Lde R, Acosta EJ, Távora FF, da Silva PM, Porto VC. Effect of repeated cycles of chemical disinfection on the roughness and hardness of hard reline acrylic resins. Gerodontology. 2010; 27:147–153.24. Philips RW. Science of Dental Materials. 9th ed. Philadelphia: W.B. Saunders Co;1991. p. 15–35.25. Ping Chaing BK. Polymers in the service of prosthetic dentistry. J Dent. 1984; 12:203–214.26. The Japan Society of Calorimetry and Thermal Analysis. Sorai M. Comprehensive Handbook of Calorimetry and Thermal Analysis. J. Wiley;2004.27. Aouachria K, Bensemra NB. Miscibility of PVC/PMMA blends by vicat softening temperature, viscometry, DSC and FTIR analysis. Polym Test. 2006; 25:1101–1108.28. Vuorinen AM, Dyer SR, Lassila LV, Vallittu PK. Effect of rigid rod polymer filler on mechanical properties of polymethyl methacrylate denture base material. Dent Mater. 2008; 24:708–713.29. Puri G, Berzins DW, Dhuru VB, Raj PA, Rambhia SK, Dhir G, Dentino AR. Effect of phosphate group addition on the properties of denture base resins. J Prosthet Dent. 2008; 100:302–308.30. Pavlinec J, Moszner N. Monomers for Adhesive Polymers, 8a Crosslinking Polymerization of Selected N-Substituted bis(Acrylamide)s for Dental Filling Materials. J Appl Polym Sci. 2009; 113:3137–3145.31. Pai RK, Ng JB, Pillai S, Bergström L, Hedin N. Temperature-induced formation of strong gels of acrylamide-based polyelectrolytes. J Colloid Interface Sci. 2009; 337:46–53.32. Hacker MC, Klouda L, Ma BB, Kretlow JD, Mikos AG. Synthesis and characterization of injectable,thermally and chemically gelable, amphiphilic poly(N-isopropylacrylamide)-based macromers. Biomacromolecules. 2008; 9:1558–1570.33. Bhattacharya A, Rawlins J, Ray P. Polymer grafting and crosslinking. Wiley;2009.34. Azevedo A, Machado AL, Vergani CE, Giampaolo ET, Pavarina AC. Hardness of denture base and hard chair-side reline acrylic resins. J Appl Oral Sci. 2005; 13:291–295.35. Rueggeberg FA, Craig RG. Correlation of parameters used to estimate monomer conversion in a light-cured composite. J Dent Res. 1988; 67:932–937.36. Rodford RA. Further development and evaluation of high impact strength denture base materials. J Dent. 1990; 18:151–157.37. Ruyter IE, Svendsen SA. Flexural properties of denture base polymers. J Prosthet Dent. 1980; 43:95–104.38. Blagojevic V, Murphy VM. Microwave polymerization of denture base materials. A comparative study. J Oral Rehabil. 1999; 26:804–808.39. Sperling LH. Introduction to physical polymer science. Wiley;1986.40. Neppelenbroek KH, Pavarina AC, Vergani CE, Giampaolo ET. Hardness of heat-polymerized acrylic resins after disinfection and long-term water immersion. J Prosthet Dent. 2005; 93:171–176.41. Rao S, Rao GK. Cure studies on bifunctional epoxy matrices using a domestic microwave oven. Polym Test. 2008; 27:645–652.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of flexural strength according to thickness between CAD/CAM denture base resins and conventional denture base resins
- Physical properties and color stability of injection-molded thermoplastic denture base resins
- Physical Properties Of Relining Denture Base Resins With Different Polymerizing Methods
- The mechanical properties of 3D printed denture base resin incorporating essential oil microcapsules
- A case of allergic contact stomatitis due to denture relining materials