J Adv Prosthodont.
2014 Oct;6(5):406-414. 10.4047/jap.2014.6.5.406.
Cellular viability and genetic expression of human gingival fibroblasts to zirconia with enamel matrix derivative (Emdogain(R))
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, School of Dentistry, Kyung Hee University, Seoul, Republic of Korea.
- 2Department of Oral Anatomy, Dental School, Kangnung-Wonju National University, Kangnung, Republic of Korea.
- 3Department of Prosthodontics and Operative Dentistry, Tufts University School of Dental Medicine, Boston, MA, USA.
- 4Department of Prosthodontics, School of Dentistry, Kyung Hee University, Seoul, Republic of Korea. ahranp@khu.ac.kr
- KMID: 2284741
- DOI: http://doi.org/10.4047/jap.2014.6.5.406
Abstract
- PURPOSE
The objective of this study was to investigate the biologic effects of enamel matrix derivative (EMD) with different concentrations on cell viability and the genetic expression of human gingival fibroblasts (HGF) to zirconia surfaces.
MATERIALS AND METHODS
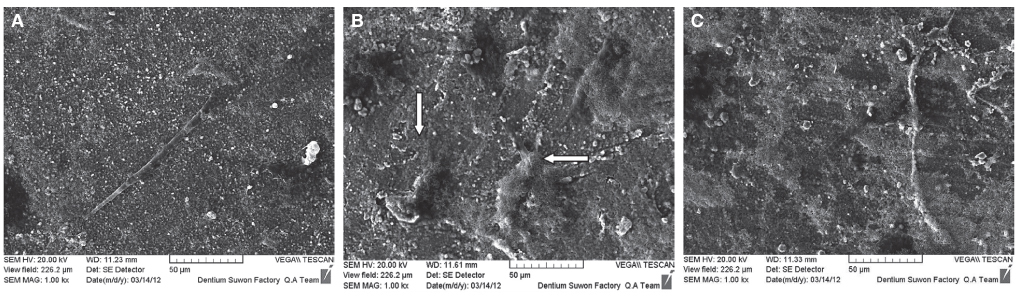
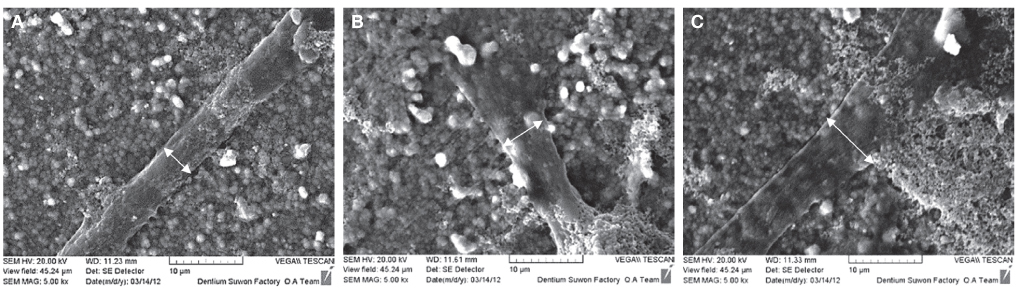
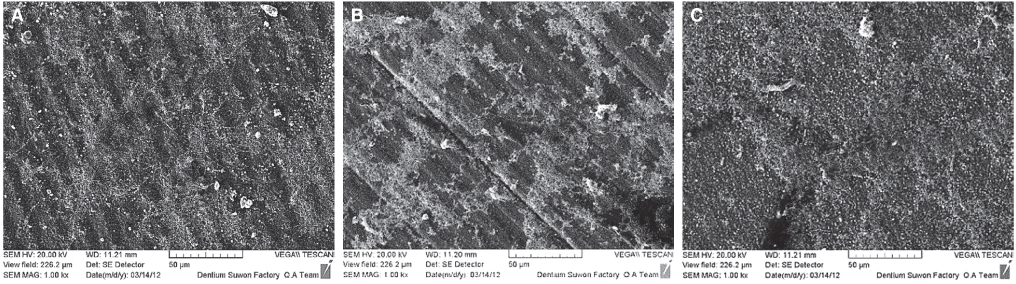
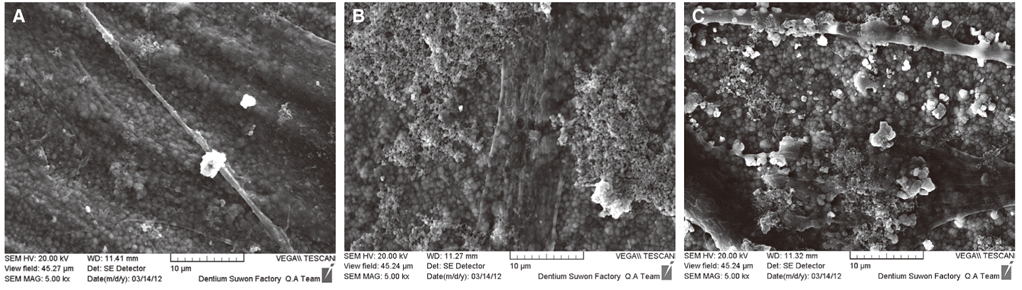
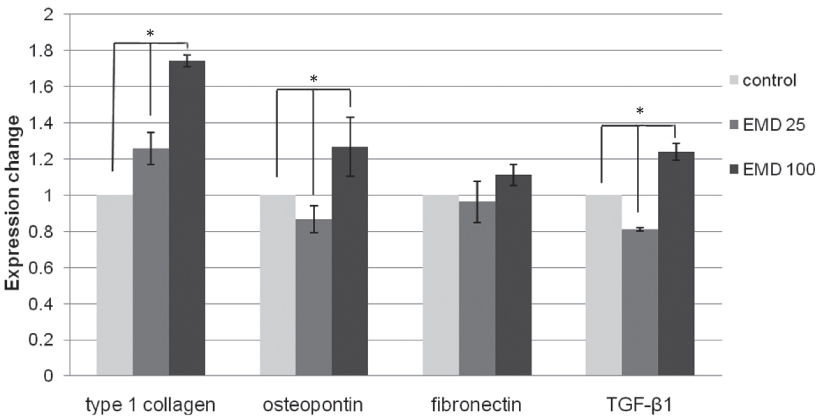
Immortalized human gingival fibroblasts (HGF) were cultured (1) without EMD, (2) with EMD 25 microg/mL, and (3) with EMD 100 microg/mL on zirconia discs. MTT assay was performed to evaluate the cell proliferation activity and SEM was carried out to examine the cellular morphology and attachment. The mRNA expression of collagen type I, osteopontin, fibronectin, and TGF-beta1 was evaluated with the real-time polymerase chain reaction (RT-PCR).
RESULTS
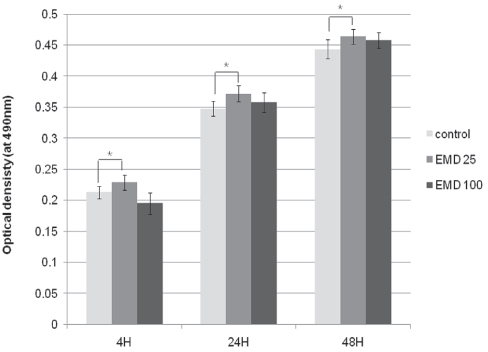
From MTT assay, HGF showed more proliferation in EMD 25 microg/mL group than control and EMD 100 microg/mL group (P<.05). HGFs showed more flattened cellular morphology on the experimental groups than on the control group after 4h culture and more cellular attachments were observed on EMD 25 microg/mL group and EMD 100 microg/mL group after 24h culture. After 48h of culture, cellular attachment was similar in all groups. The mRNA expression of type I collagen increased in a concentration dependent manner. The genetic expression of osteopontin, fibronectin, and TGF-beta1 was increased at EMD 100 microg/mL. However, the mRNA expression of proteins associated with cellular attachment was decreased at EMD 25 microg/mL.
CONCLUSION
Through this short term culture of HGF on zirconium discs, we conclude that EMD affects the proliferation, attachment, and cell morphology of HGF cells. Also, EMD stimulates production of extracellular matrix collagen, osteopontin, and TGF-beta1 in high concentration levels. CLINICAL RELEVANCE: With the use of EMD, protective barrier between attached gingiva and transmucosal zirconia abutment may be enhanced leading to final esthetic results with implants.
Keyword
MeSH Terms
-
Cell Proliferation
Cell Survival
Collagen
Collagen Type I
Dental Enamel*
Extracellular Matrix
Fibroblasts*
Fibronectins
Gingiva
Humans
Osteopontin
Real-Time Polymerase Chain Reaction
RNA, Messenger
Transforming Growth Factor beta1
Zirconium
Collagen
Collagen Type I
Fibronectins
Osteopontin
RNA, Messenger
Transforming Growth Factor beta1
Zirconium
Figure
Reference
-
1. Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999; 20:1–25.2. Manicone PF, Rossi Iommetti P, Raffaelli L. An overview of zirconia ceramics: basic properties and clinical applications. J Dent. 2007; 35:819–826.3. Butz F, Heydecke G, Okutan M, Strub JR. Survival rate, fracture strength and failure mode of ceramic implant abutments after chewing simulation. J Oral Rehabil. 2005; 32:838–843.4. Scarano A, Piattelli M, Caputi S, Favero GA, Piattelli A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: an in vivo human study. J Periodontol. 2004; 75:292–296.5. Rimondini L, Cerroni L, Carrassi A, Torricelli P. Bacterial colonization of zirconia ceramic surfaces: an in vitro and in vivo study. Int J Oral Maxillofac Implants. 2002; 17:793–798.6. Degidi M, Artese L, Scarano A, Perrotti V, Gehrke P, Piattelli A. Inflammatory infiltrate, microvessel density, nitric oxide synthase expression, vascular endothelial growth factor expression, and proliferative activity in peri-implant soft tissues around titanium and zirconium oxide healing caps. J Periodontol. 2006; 77:73–80.7. Welander M, Abrahamsson I, Berglundh T. The mucosal barrier at implant abutments of different materials. Clin Oral Implants Res. 2008; 19:635–641.8. van Brakel R, Cune MS, van Winkelhoff AJ, de Putter C, Verhoeven JW, van der Reijden W. Early bacterial colonization and soft tissue health around zirconia and titanium abutments: an in vivo study in man. Clin Oral Implants Res. 2011; 22:571–577.9. Hammarström L. Enamel matrix, cementum development and regeneration. J Clin Periodontol. 1997; 24:658–668.10. Hammarström L, Heijl L, Gestrelius S. Periodontal regeneration in a buccal dehiscence model in monkeys after application of enamel matrix proteins. J Clin Periodontol. 1997; 24:669–677.11. Gestrelius S, Andersson C, Johansson AC, Persson E, Brodin A, Rydhag L, Hammarström L. Formulation of enamel matrix derivative for surface coating. Kinetics and cell colonization. J Clin Periodontol. 1997; 24:678–684.12. Gestrelius S, Lyngstadaas SP, Hammarström L. Emdogain-periodontal regeneration based on biomimicry. Clin Oral Investig. 2000; 4:120–125.13. Sculean A, Windisch P, Keglevich T, Fabi B, Lundgren E, Lyngstadaas PS. Presence of an enamel matrix protein derivative on human teeth following periodontal surgery. Clin Oral Investig. 2002; 6:183–187.14. Van der Pauw MT, Van den Bos T, Everts V, Beertsen W. Enamel matrix-derived protein stimulates attachment of periodontal ligament fibroblasts and enhances alkaline phosphatase activity and transforming growth factor beta1 release of periodontal ligament and gingival fibroblasts. J Periodontol. 2000; 71:31–43.15. Lyngstadaas SP, Lundberg E, Ekdahl H, Andersson C, Gestrelius S. Autocrine growth factors in human periodontal ligament cells cultured on enamel matrix derivative. J Clin Periodontol. 2001; 28:181–188.16. Jiang J, Goodarzi G, He J, Li H, Safavi KE, Spångberg LS, Zhu Q. Emdogain-gel stimulates proliferation of odontoblasts and osteoblasts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 102:698–702.17. He J, Jiang J, Safavi KE, Spångberg LS, Zhu Q. Emdogain promotes osteoblast proliferation and differentiation and stimulates osteoprotegerin expression. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004; 97:239–245.18. Lossdörfer S, Sun M, Götz W, Dard M, Jäger A. Enamel matrix derivative promotes human periodontal ligament cell differentiation and osteoprotegerin production in vitro. J Dent Res. 2007; 86:980–985.19. Jue SS, Lee WY, Kwon YD, Kim YR, Pae A, Lee B. The effects of enamel matrix derivative on the proliferation and differentiation of human mesenchymal stem cells. Clin Oral Implants Res. 2010; 21:741–746.20. Heden G, Wennström J, Lindhe J. Periodontal tissue alterations following Emdogain treatment of periodontal sites with angular bone defects. A series of case reports. J Clin Periodontol. 1999; 26:855–860.21. Trombelli L, Bottega S, Zucchelli G. Supracrestal soft tissue presentation with enamel matrix proteins in the treatment of deep intrabony defects. A report of 35 consecutively treated cases. J Clin Periodontol. 2002; 29:433–439.22. Yilmaz S, Kuru B, Altuna-Kiraç E. Enamel matrix proteins in the treatment of periodontal sites with horizontal type of bone loss. J Clin Periodontol. 2003; 30:197–206.23. Sculean A, Chiantella GC, Arweiler NB, Becker J, Schwarz F, Stavropoulos A. Five-year clinical and histologic results following treatment of human intrabony defects with an enamel matrix derivative combined with a natural bone mineral. Int J Periodontics Restorative Dent. 2008; 28:153–161.24. Sculean A, Kiss A, Miliauskaite A, Schwarz F, Arweiler NB, Hannig M. Ten-year results following treatment of intra-bony defects with enamel matrix proteins and guided tissue regeneration. J Clin Periodontol. 2008; 35:817–824.25. Modica F, Del Pizzo M, Roccuzzo M, Romagnoli R. Coronally advanced flap for the treatment of buccal gingival recessions with and without enamel matrix derivative. A splitmouth study. J Periodontol. 2000; 71:1693–1698.26. Berlucchi I, Francetti L, Del Fabbro M, Testori T, Weinstein RL. Enamel matrix proteins (Emdogain) in combination with coronally advanced flap or subepithelial connective tissue graft in the treatment of shallow gingival recessions. Int J Periodontics Restorative Dent. 2002; 22:583–593.27. Hägewald S, Spahr A, Rompola E, Haller B, Heijl L, Bernimoulin JP. Comparative study of Emdogain and coronally advanced flap technique in the treatment of human gingival recessions. A prospective controlled clinical study. J Clin Periodontol. 2002; 29:35–41.28. Nemcovsky CE, Artzi Z, Tal H, Kozlovsky A, Moses O. A multicenter comparative study of two root coverage procedures: coronally advanced flap with addition of enamel matrix proteins and subpedicle connective tissue graft. J Periodontol. 2004; 75:600–607.29. Cueva MA, Boltchi FE, Hallmon WW, Nunn ME, Rivera-Hidalgo F, Rees T. A comparative study of coronally advanced flaps with and without the addition of enamel matrix derivative in the treatment of marginal tissue recession. J Periodontol. 2004; 75:949–956.30. Keila S, Nemcovsky CE, Moses O, Artzi Z, Weinreb M. In vitro effects of enamel matrix proteins on rat bone marrow cells and gingival fibroblasts. J Dent Res. 2004; 83:134–138.31. Zeldich E, Koren R, Nemcovsky C, Weinreb M. Enamel matrix derivative stimulates human gingival fibroblast proliferation via ERK. J Dent Res. 2007; 86:41–46.32. Mustafa K, Silva Lopez B, Hultenby K, Wennerberg A, Arvidson K. Attachment and proliferation of human oral fibroblasts to titanium surfaces blasted with TiO2 particles. A scanning electron microscopic and histomorphometric analysis. Clin Oral Implants Res. 1998; 9:195–207.33. Grössner-Schreiber B, Herzog M, Hedderich J, Dück A, Hannig M, Griepentrog M. Focal adhesion contact formation by fibroblasts cultured on surface-modified dental implants: an in vitro study. Clin Oral Implants Res. 2006; 17:736–745.34. Zhang F, Huang Y, Li X, Zhao S. Surface modification and its effect on attachment, spreading, and proliferation of human gingival fibroblasts. Int J Oral Maxillofac Implants. 2011; 26:1183–1192.35. Pae A, Lee H, Kim HS, Kwon YD, Woo YH. Attachment and growth behaviour of human gingival fibroblasts on titanium and zirconia ceramic surfaces. Biomed Mater. 2009; 4:025005.36. Yamano S, Ma AK, Shanti RM, Kim SW, Wada K, Sukotjo C. The influence of different implant materials on human gingival fibroblast morphology, proliferation, and gene expression. Int J Oral Maxillofac Implants. 2011; 26:1247–1255.37. Abrahamsson I, Berglundh T, Glantz PO, Lindhe J. The mucosal attachment at different abutments. An experimental study in dogs. J Clin Periodontol. 1998; 25:721–727.38. Glauser R, Sailer I, Wohlwend A, Studer S, Schibli M, Schärer P. Experimental zirconia abutments for implant-supported single-tooth restorations in esthetically demanding regions: 4-year results of a prospective clinical study. Int J Prosthodont. 2004; 17:285–290.39. Canullo L. Clinical outcome study of customized zirconia abutments for single-implant restorations. Int J Prosthodont. 2007; 20:489–493.40. Zembic A, Sailer I, Jung RE, Hämmerle CH. Randomized-controlled clinical trial of customized zirconia and titanium implant abutments for single-tooth implants in canine and posterior regions: 3-year results. Clin Oral Implants Res. 2009; 20:802–808.41. Raffaelli L, Rossi Iommetti P, Piccioni E, Toesca A, Serini S, Resci F, Missori M, De Spirito M, Manicone PF, Calviello G. Growth, viability, adhesion potential, and fibronectin expression in fibroblasts cultured on zirconia or feldspatic ceramics in vitro. J Biomed Mater Res A. 2008; 86:959–968.42. Tetè S, Mastrangelo F, Bianchi A, Zizzari V, Scarano A. Collagen fiber orientation around machined titanium and zirconia dental implant necks: an animal study. Int J Oral Maxillofac Implants. 2009; 24:52–58.43. Rincon JC, Haase HR, Bartold PM. Effect of Emdogain on human periodontal fibroblasts in an in vitro wound-healing model. J Periodontal Res. 2003; 38:290–295.44. Gestrelius S, Andersson C, Lidström D, Hammarström L, Somerman M. In vitro studies on periodontal ligament cells and enamel matrix derivative. J Clin Periodontol. 1997; 24:685–692.45. Giannopoulou C, Cimasoni G. Functional characteristics of gingival and periodontal ligament fibroblasts. J Dent Res. 1996; 75:895–902.46. Ivanovski S, Li H, Haase HR, Bartold PM. Expression of bone associated macromolecules by gingival and periodontal ligament fibroblasts. J Periodontal Res. 2001; 36:131–141.47. Carmona-Rodríguez B, Alvarez-Pérez MA, Narayanan AS, Zeichner-David M, Reyes-Gasga J, Molina-Guarneros J, García-Hernández AL, Suárez-Franco JL, Chavarría IG, Villarreal-Ramírez E, Arzate H. Human Cementum Protein 1 induces expression of bone and cementum proteins by human gingival fibroblasts. Biochem Biophys Res Commun. 2007; 358:763–769.48. van der Pauw MT, Van den Bos T, Everts V, Beertsen W. Phagocytosis of fibronectin and collagens type I, III, and V by human gingival and periodontal ligament fibroblasts in vitro. J Periodontol. 2001; 72:1340–1347.49. Heino J, Ignotz RA, Hemler ME, Crouse C, Massagué J. Regulation of cell adhesion receptors by transforming growth factor-beta. Concomitant regulation of integrins that share a common beta 1 subunit. J Biol Chem. 1989; 264:380–388.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Enamel Matrix Derivative and Calcium Sulfate Paste on the Healing of 1-Wall Intrabony Defects in Beagle Dogs
- Effects of enamel matrix protein derivatives on the periodontal ligament like fibroblast and osteoblast like cells
- Enamel matrix derivative for replanted teeth in animal models: a systematic review and meta-analysis
- Effects of Glycosaminoglycan on the Growth of Human Gingival Fibroblast
- Effects of Replicative Senescence on the Cell Cycle Regulation in Human Gingival Fibroblasts