Diabetes Metab J.
2011 Feb;35(1):72-79. 10.4093/dmj.2011.35.1.72.
Repeated Gene Transfection Impairs the Engraftment of Transplanted Porcine Neonatal Pancreatic Cells
- Affiliations
-
- 1Research Institute of Immunobiology, Department of Biomedical Sciences, The Catholic University of Korea School of Medicine, Seoul, Korea. sukklee@catholic.ac.kr
- 2Division of Endocrinology & Metabolism, Department of Internal Medicine, The Catholic University of Korea School of Medicine, Seoul, Korea.
- KMID: 2281495
- DOI: http://doi.org/10.4093/dmj.2011.35.1.72
Abstract
- BACKGROUND
Previously, we reported that neonatal porcine pancreatic cells transfected with hepatocyte growth factor (HGF) gene in an Epstein-Barr virus (EBV)-based plasmid (pEBVHGF) showed improved proliferation and differentiation compared to those of the control. In this study, we examined if pancreatic cells transfected repeatedly with pEBVHGF can be successfully grafted to control blood glucose in a diabetes mouse model.
METHODS
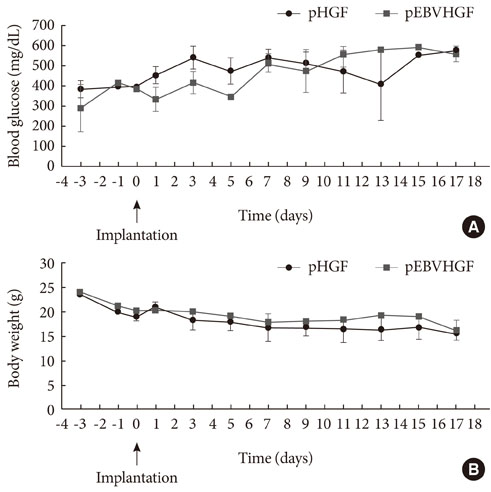
Neonatal porcine pancreatic cells were cultured as a monolayer and were transfected with pEBVHGF every other day for a total of three transfections. The transfected pancreatic cells were re-aggregated and transplanted into kidney capsules of diabetic nude mice or normal nude mice. Blood glucose level and body weight were measured every other day after transplantation. The engraftment of the transplanted cells and differentiation into beta cells were assessed using immunohistochemistry.
RESULTS
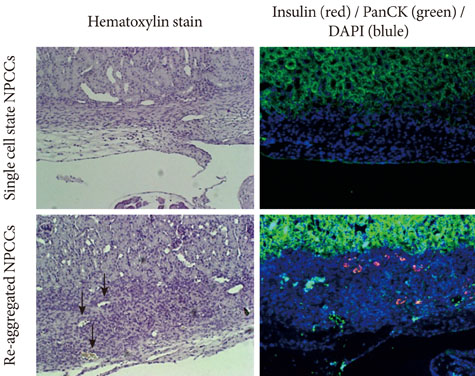
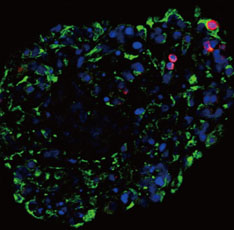
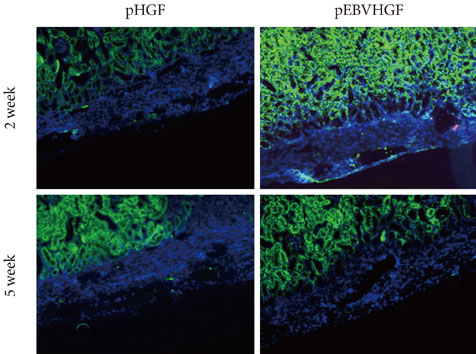
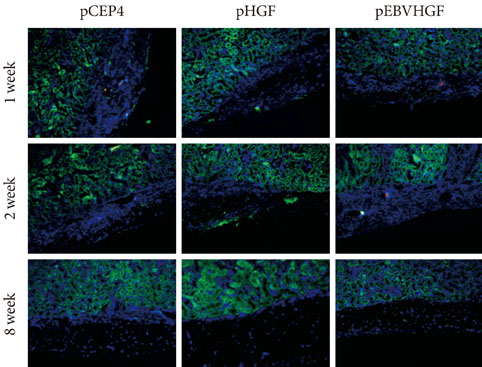
Re-aggregation of the pancreatic cells before transplantation improved engraftment of the cells and facilitated neovascularization of the graft. Right before transplantation, pancreatic cells that were transfected with pEBVHGF and then re-aggregated showed ductal cell marker expression. However, ductal cells disappeared and the cells underwent fibrosis in a diabetes mouse model two to five weeks after transplantation; these mice also did not show controlled blood glucose levels. Furthermore, pancreatic cells transplanted into nude mice with normal blood glucose showed poor graft survival regardless of the type of transfected plasmid (pCEP4, pHGF, or pEBVHGF).
CONCLUSION
For clinical application of transfected neonatal porcine pancreatic cells, further studies are required to develop methods of overcoming the damage for the cells caused by repeated transfection and to re-aggregate them into islet-like structures.
Keyword
MeSH Terms
Figure
Reference
-
1. Cavanagh TJ, Lakey JR, Wright MJ, Albertson T, Wile K, Fetterhoff TJ. Identification of a pig strain with maximal islet mass. Transplant Proc. 1998. 30:368.2. Ohgawara H, Iwanaga T, Yui R, Nishijima S, Hirata Y. Monolayer-forming islet cell culture from neonatal pig pancreas: using sequential treatment with EDTA-dispase and monoiodoacetic acid for preparation and purification. Tohoku J Exp Med. 1987. 153:375–382.3. Witzigmann H, Ludwig S, Armann B, Gabel G, Teupser D, Kratzsch J, Pietsch UC, Tannapfel A, Geissler F, Hauss J, Uhlmann D. Endothelin(A) receptor blockade reduces ischemia/reperfusion injury in pig pancreas transplantation. Ann Surg. 2003. 238:264–274.4. Omer A, Duvivier-Kali VF, Trivedi N, Wilmot K, Bonner-Weir S, Weir GC. Survival and maturation of microencapsulated porcine neonatal pancreatic cell clusters transplanted into immunocompetent diabetic mice. Diabetes. 2003. 52:69–75.5. Ohgawara H, Kobayashi A, Kawamura M, Karibe S, Fu Q, Omori Y, Akaike T. Development of a method for embedded-culture of pig pancreatic islet-like cell clusters in agarose containing maltose-carrying polystyrene (HEVM) and nicotinamide. Cell Transplant. 1994. 3:83–89.6. Chen B, Liu J, Zhu X, Liu X. Transplantation of encapsulated neonatal porcine islet-like cluster cells into diabetic rats. Chin Med J (Engl). 1996. 109:197–200.7. Korsgren O, Jansson L, Sundler F. Reinnervation of transplanted fetal porcine endocrine pancreas. Evidence for initial growth and subsequent degeneration of nerve fibers in the islet grafts. Transplantation. 1996. 62:352–357.8. Yoon KH, Quickel RR, Tatarkiewicz K, Weir GC, Bonner-Weir S. Growth and differentiation of transplanted porcine neonatal pancreatic cell clusters in normal nude mice. Transplant Proc. 1998. 30:601.9. Weir GC, Quickel RR, Yoon KH, Tatarkiewicz K, Ulrich TR, Hollister-Lock J, Bonner-Weir S. Porcine neonatal pancreatic cell clusters (NPCCs): a potential source of tissue for islet transplantation. Ann Transplant. 1997. 2:63–68.10. Korbutt GS, Elliott JF, Ao Z, Smith DK, Warnock GL, Rajotte RV. Large scale isolation, growth, and function of porcine neonatal islet cells. J Clin Invest. 1996. 97:2119–2129.11. Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group. Effect of intensive diabetes treatment on carotid artery wall thickness in the epidemiology of diabetes interventions and complications. Diabetes. 1999. 48:383–390.12. Tatarkiewicz K, Lopez-Avalos MD, Yoon KH, Trivedi N, Quickel RR, Bonner-Weir S, Weir GC. Development and retroviral transduction of porcine neonatal pancreatic islet cells in monolayer culture. Dev Growth Differ. 2003. 45:39–50.13. Lopez-Avalos MD, Tatarkiewicz K, Sharma A, Bonner-Weir S, Weir GC. Enhanced maturation of porcine neonatal pancreatic cell clusters with growth factors fails to improve transplantation outcome. Transplantation. 2001. 71:1154–1162.14. Valdes-Gonzalez RA, Dorantes LM, Garibay GN, Bracho-Blanchet E, Mendez AJ, Davila-Perez R, Elliott RB, Teran L, White DJ. Xenotransplantation of porcine neonatal islets of Langerhans and Sertoli cells: a 4-year study. Eur J Endocrinol. 2005. 153:419–427.15. Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006. 212:167–178.16. Nakamura T, Nawa K, Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984. 122:1450–1459.17. Nakamura T. Structure and function of hepatocyte growth factor. Prog Growth Factor Res. 1991. 3:67–85.18. Boros P, Miller CM. Hepatocyte growth factor: a multifunctional cytokine. Lancet. 1995. 345:293–295.19. Guido L, Basta G, Racanicchi L, Mancuso F, Luca G, Macchiarulo G, Brunetti P, Calafiore R. Short-term stimulation studies on neonatal pig pancreatic duct-derived cell monolayers. Transplant Proc. 2005. 37:2715–2718.20. Kim MS, Kim JW, Sun C, Oh ST, Yoon KH, Lee SK. Induction of efficient differentiation and survival of porcine neonatal pancreatic cell clusters using an EBV-based plasmid expressing HGF. J Biochem. 2008. 143:497–503.21. Nanbo A, Sugden A, Sugden B. The coupling of synthesis and partitioning of EBV's plasmid replicon is revealed in live cells. EMBO J. 2007. 26:4252–4262.22. Hauge-Evans AC, Squires PE, Persaud SJ, Jones PM. Pancreatic beta-cell-to-beta-cell interactions are required for integrated responses to nutrient stimuli: enhanced Ca2+ and insulin secretory responses of MIN6 pseudoislets. Diabetes. 1999. 48:1402–1408.23. Sun CL, Ham DS, Park HS, Kim JW, Cho JH, Song KH, Son HY, Yoon KH. Rapamycin suppresses the expansion and differentiation of porcine neonatal pancreas cell clusters. Transplantation. 2010. 90:717–724.24. Trivedi N, Hollister-Lock J, Lopez-Avalos MD, O'Neil JJ, Keegan M, Bonner-Weir S, Weir GC. Increase in beta-cell mass in transplanted porcine neonatal pancreatic cell clusters is due to proliferation of beta-cells and differentiation of duct cells. Endocrinology. 2001. 142:2115–2122.25. Ko SH, Kwon HS, Suh SH, Yang JH, Kim SR, Ahn YB, Song KH, Yoo SJ, Son HS, Cha BY, Lee KW, Son HY, Kang SK, Park CG, Yoon KH. Dexamethasone suppresses the expansion and transdifferentiation of transplanted porcine neonatal pancreas cell clusters (NPCCs) into beta-cells in normal nude mice. Diabetes Res Clin Pract. 2004. 66:Suppl 1. S97–S101.26. Yoon KH, Quickel RR, Tatarkiewicz K, Ulrich TR, Hollister-Lock J, Trivedi N, Bonner-Weir S, Weir GC. Differentiation and expansion of beta cell mass in porcine neonatal pancreatic cell clusters transplanted into nude mice. Cell Transplant. 1999. 8:673–689.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adenoviruses Expressing PDX-1, BETA2/NeuroD and MafA Induces the Transdifferentiation of Porcine Neonatal Pancreas Cell Clusters and Adult Pig Pancreatic Cells into Beta-Cells
- Expression of Gal alpha1,3 Gal Antigen and Galactosyl Transferase mRNA in Porcine Neonatal Pancreatic Tissue
- In Vitro Expansion and Differentiation of Islet Precursor Cells from Cultured Neonatal Porcine Pancreatic Tissue
- Study for the Improvement of Early Implantation and Long Term Graft Survival in Pancreatic Islet Cell Transplantation by Induction of Angiogenesis with Gene Transfection of Vascuar Endothelial Growth Factor
- Cotransplantation of Cord Blood Hematopoietic Stem Cells and Culture-Expanded and GM-CSF-/SCF-Transfected Mesenchymal Stem Cells in SCID Mice