Diabetes Metab J.
2014 Apr;38(2):100-106. 10.4093/dmj.2014.38.2.100.
The Role of Heat Shock Response in Insulin Resistance and Diabetes
- Affiliations
-
- 1Department of Metabolic Medicine, Kumamoto University Faculty of Life Sciences, Kumamoto, Japan. t-kondo@gpo.kumamoto-u.ac.jp
- 2Department of Molecular Medicine, Kumamoto University Faculty of Life Sciences, Kumamoto, Japan.
- KMID: 2280700
- DOI: http://doi.org/10.4093/dmj.2014.38.2.100
Abstract
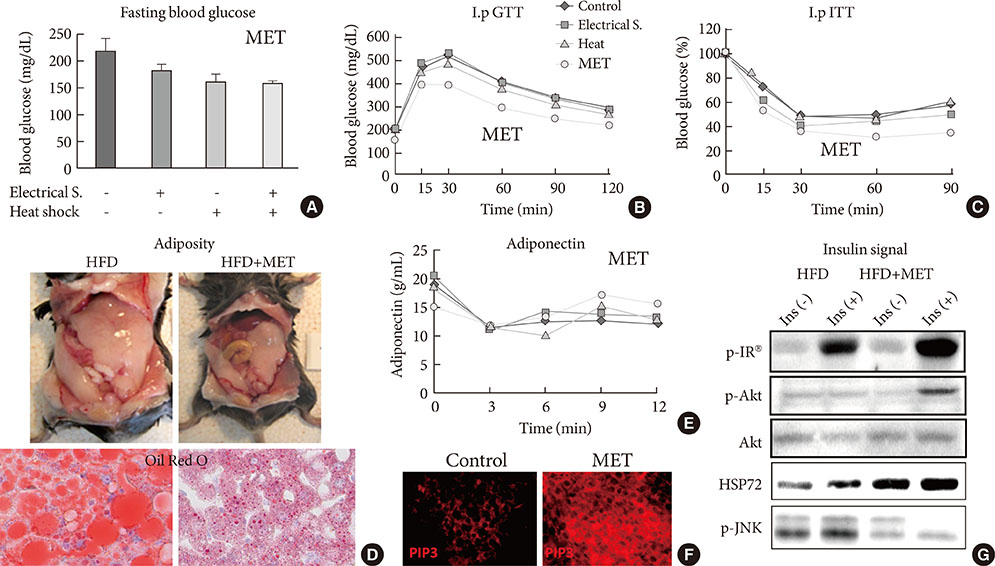
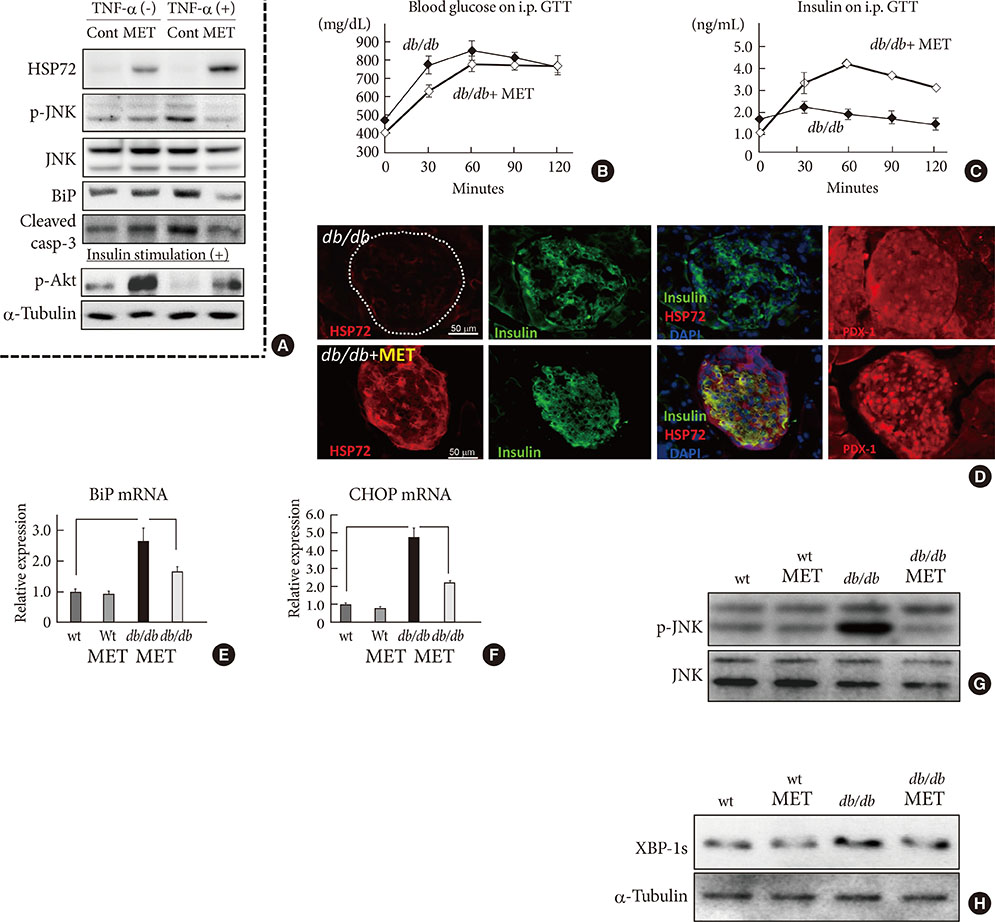
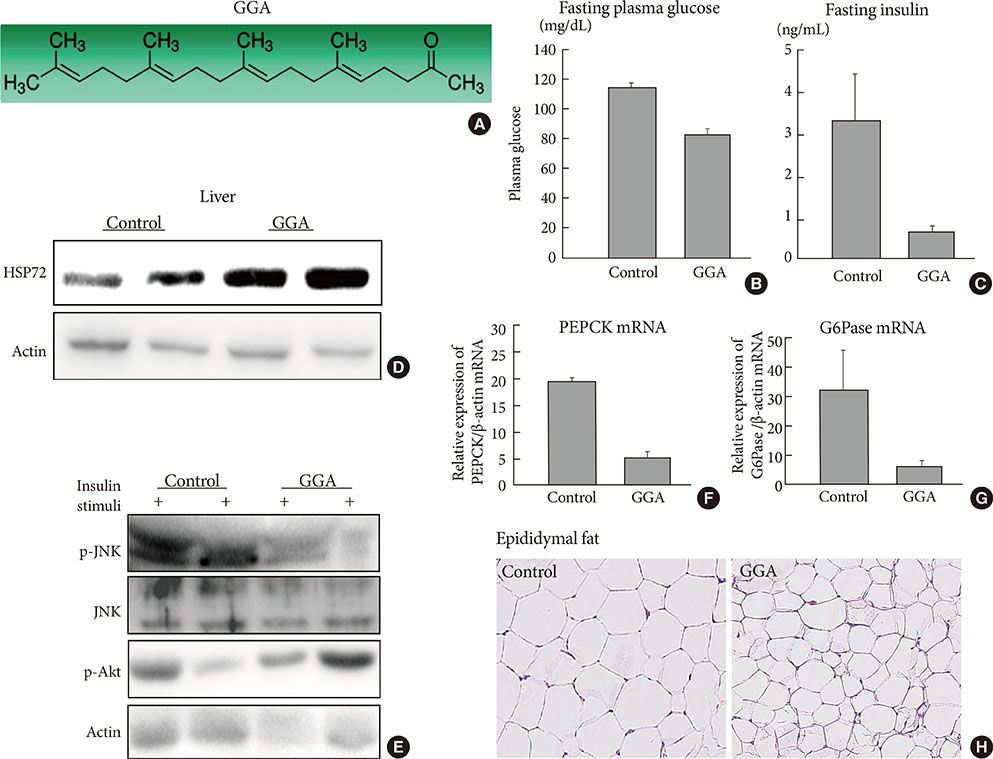
- The expansion of life-style related diseases, such as metabolic syndrome (MS) and type 2 diabetes mellitus (T2DM), appears to be unstoppable. It is also difficult to cease their complications in spite of many antidiabetic medications or intervention of public administration. We and our collaborators found that physical medicine using simultaneous stimulation of heat with mild electric current activates heat shock response, thereby reducing visceral adiposity, insulin resistance, chronic inflammation and improving glucose homeostasis in mice models of T2DM, as well as in humans with MS or T2DM. This combination therapy exerts novel action on insulin signaling, beta-cell protection and body compositions, and may provide a new therapeutic alternative in diabetic treatment strategy.
MeSH Terms
Figure
Reference
-
1. Ueki K, Kondo T, Kahn CR. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol. 2004; 24:5434–5446.2. Ritossa F. A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia. 1962; 18:571–573.3. Adachi H, Kondo T, Ogawa R, Sasaki K, Morino-Koga S, Sakakida M, Kawashima J, Motoshima H, Furukawa N, Tsuruzoe K, Miyamura N, Kai H, Araki E. An acylic polyisoprenoid derivative, geranylgeranylacetone protects against visceral adiposity and insulin resistance in high-fat-fed mice. Am J Physiol Endocrinol Metab. 2010; 299:E764–E771.4. Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, Horvath I, Mestril R, Watt MJ, Hooper PL, Kingwell BA, Vigh L, Hevener A, Febbraio MA. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2008; 105:1739–1744.5. Gupte AA, Bomhoff GL, Morris JK, Gorres BK, Geiger PC. Lipoic acid increases heat shock protein expression and inhibits stress kinase activation to improve insulin signaling in skeletal muscle from high-fat-fed rats. J Appl Physiol (1985). 2009; 106:1425–1434.6. Gupte AA, Bomhoff GL, Swerdlow RH, Geiger PC. Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes. 2009; 58:567–578.7. Kokura S, Adachi S, Manabe E, Mizushima K, Hattori T, Okuda T, Nakabe N, Handa O, Takagi T, Naito Y, Yoshida N, Yoshikawa T. Whole body hyperthermia improves obesity-induced insulin resistance in diabetic mice. Int J Hyperthermia. 2007; 23:259–265.8. Morino S, Kondo T, Sasaki K, Adachi H, Suico MA, Sekimoto E, Matsuda T, Shuto T, Araki E, Kai H. Mild electrical stimulation with heat shock ameliorates insulin resistance via enhanced insulin signaling. PLoS One. 2008; 3:e4068.9. Kavanagh K, Flynn DM, Jenkins KA, Zhang L, Wagner JD. Restoring HSP70 deficiencies improves glucose tolerance in diabetic monkeys. Am J Physiol Endocrinol Metab. 2011; 300:E894–E901.10. Kondo T, Sasaki K, Adachi H, Nakayama Y, Hatemura M, Matsuyama R, Tsuruzoe K, Furukawa N, Motoshima H, Morino Koga S, Yamashita Y, Miyamura N, Kai H, Araki E. Heat shock treatment with mild electrical stimulation safely reduced inflammatory markers in healthy male subjects. Obes Res Clin Pract. 2010; 4:e83–e162.11. Hooper PL. Hot-tub therapy for type 2 diabetes mellitus. N Engl J Med. 1999; 341:924–925.12. Chow AM, Steel R, Anderson RL. Hsp72 chaperone function is dispensable for protection against stress-induced apoptosis. Cell Stress Chaperones. 2009; 14:253–263.13. Literáti-Nagy B, Kulcsar E, Literati-Nagy Z, Buday B, Peterfai E, Horvath T, Tory K, Kolonics A, Fleming A, Mandl J, Korányi L. Improvement of insulin sensitivity by a novel drug, BGP-15, in insulin-resistant patients: a proof of concept randomized double-blind clinical trial. Horm Metab Res. 2009; 41:374–380.14. Kondo T, Sasaki K, Matsuyama R, Morino-Koga S, Adachi H, Suico MA, Kawashima J, Motoshima H, Furukawa N, Kai H, Araki E. Hyperthermia with mild electrical stimulation protects pancreatic beta-cells from cell stresses and apoptosis. Diabetes. 2012; 61:838–847.15. Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, Penninger JM. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006; 442:457–460.16. Morino-Koga S, Yano S, Kondo T, Shimauchi Y, Matsuyama S, Okamoto Y, Suico MA, Koga T, Sato T, Shuto T, Arima H, Wada I, Araki E, Kai H. Insulin receptor activation through its accumulation in lipid rafts by mild electrical stress. J Cell Physiol. 2013; 228:439–446.17. Yano S, Morino-Koga S, Kondo T, Suico MA, Koga T, Shimauchi Y, Matsuyama S, Shuto T, Sato T, Araki E, Kai H. Glucose uptake in rat skeletal muscle L6 cells is increased by low-intensity electrical current through the activation of the phosphatidylinositol-3-OH kinase (PI-3K) / Akt pathway. J Pharmacol Sci. 2011; 115:94–98.18. Morino S, Suico MA, Kondo T, Sekimoto E, Yano S, Matsuda T, Matsuno T, Shuto T, Araki E, Kai H. Mild electrical stimulation increases ubiquitinated proteins and Hsp72 in A549 cells via attenuation of proteasomal degradation. J Pharmacol Sci. 2008; 108:222–226.19. Hooper PL, Hooper PL. Inflammation, heat shock proteins, and type 2 diabetes. Cell Stress Chaperones. 2009; 14:113–115.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Using Motivational Interviewing to Overcome Psychological Insulin Resistance
- Psychological Insulin Resistance: Key Factors and Intervention
- Rho-kinase and Insulin Signaling
- Glut4 in the insulin resistance of NIDDM
- Response: Effects of Aerobic Exercise Intensity on Insulin Resistance in Patients with Type 2 Diabetes Mellitus (Korean Diabetes J 33:(5)401-411, 2009)