Korean J Pediatr Infect Dis.
2013 Dec;20(3):123-130.
Measles Viral Infection in PD-1 Gene Knockout Mice
- Affiliations
-
- 1Department of Pediatrics, Yonsei University College of Medicine, Wonju, Korea.
- 2Department of Pediatrics, Yonsei University College of Medicine, Korea. dskim6634@yuhs.ac
- 3Department of Pharmacology, Kwandong University College of Medicine, Korea.
Abstract
- PURPOSE
Subacute sclerosing panencephalitis (SSPE) is a neurodegerative disease due to persistent measles virus infection. We investigated the role of programmed death-1 (PD-1) molecule which is related with chronic viral infection in developing SSPE in mouse.
METHODS
We adopt the PD-1-/-, PD-1-/+, and wild type BALB/c 3 week old mice to make an animal model of SSPE by injecting measles virus (SSPE strain) intraventricularly. Three months after infusion of virus, the mice were sacrificed and examined if the typical pathologic lesions had been progressed. The sera were collected from each group of mice and the serum level of IL-21 was measured with ELISA kit.
RESULTS
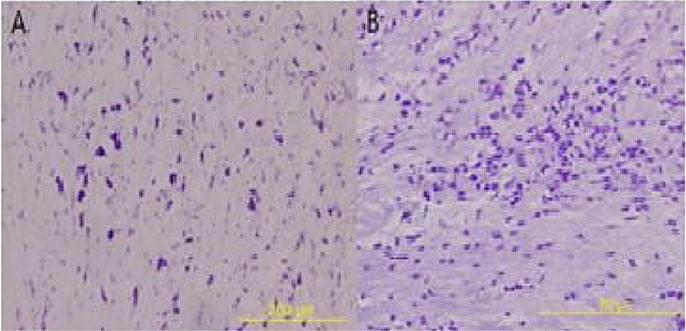
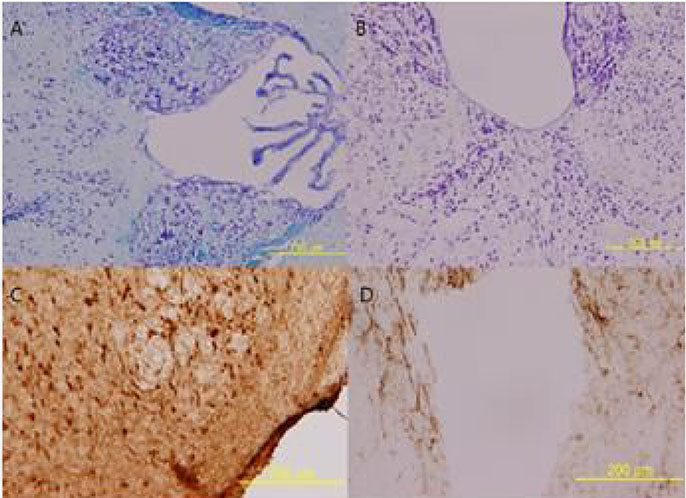
The necrotic lesions on white matter and gliosis were found in focal areas in wild type BALB/c. The extent of lesion was smaller in heterotype BALB/c. Scanty lesions were found in PD-1-/- mice. The sera level of IL-21 was not elevated in all three groups.
CONCLUSION
Our data suggest that the PD-1 molecule may play a role in persistent viral infection.
MeSH Terms
Figure
Reference
-
1. van den Ent MM, Brown DW, Hoekstra EJ, Christie A, Cochi SL. Measles mortality reduction contributes substantially to reduction of all cause mortality among children less than five years of age, 1990-2008. J Infect Dis. 2011; 204:Suppl 1. S18–S23.
Article2. Oldstone MB, Dales S, Tishon A, Lewicki H, Martin L. A role for dual viral hits in causation of subacute sclerosing panencephalitis. J Exp Med. 2005; 202:1185–1190.
Article3. Garg RK. Subacute sclerosing panencephalitis. J Neurol. 2008; 255:1861–1871.
Article4. Schneider-Schaulies S, ter Meulen V. Pathogenic aspects of measles virus infections. Arch Virol Suppl. 1999; 15:139–158.
Article5. Gascon GG. Randomized treatment study of inosiplex versus combined inosiplex and intraventrcular interferon-alpha in subacute sclerosing panencephalitis (SSPE): International multicenter study. J child Neurol. 2003; 18:819–827.
Article6. Eroglu E, Gokcil Z, Bek S, Ulas UH, Ozdag MF, Odabasi Z. Long-term follow-up of patients with adult-onset subacute sclerosing panencephalitis. J Neurol Sci. 2008; 275:113–116.
Article7. Cathomen T, Mrkic B, Spehner D, Drillien R, Naef R, Pavlovic J, et al. A matrix-less measles virus is infectious and elicits extensive cell fusion: consequences for propagation in the brain. EMBO J. 1998; 17:3899–3908.
Article8. Moulin E, Beal V, Jeantet D, Horvat B, Wild TF, Waku-Kouomou D. Molecular characterization of measles virus strains causing subacute sclerosing panencephalitis in France in 1977 and 2007. J Med Virol. 2011; 83:1614–1623.
Article9. Forcic D, Baricevic M, Zgorelec R, Kruzic V, Kaic B, Marina BM, et al. Detection and characterization of measles virus strains in cases of subacute sclerosing panencephalitis in Croatia. Virus Res. 2004; 99:51–56.
Article10. Wong TC, Ayata M, Ueda S, Hirano A. Role of biased hypermutation in evolution of subacute sclerosing panencephalitis virus from progenitor acute measles virus. J Virol. 1991; 65:2191–2199.
Article11. Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, et al. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008; 205:543–555.
Article12. Macatangay BJ, Rinaldo CR. PD-1 blockade: A promising immunotherapy for HIV? Cellscience. 2009; 5:61–65.13. Reuter D, Schneider-Schaulies J. Measles virus infection of the CNS: human disease, animal models, and approaches to therapy. Med Microbiol Immunol. 2010; 199:261–271.
Article14. Oldstone MB. Modeling subacute sclerosing panencephalitis in a transgenic mouse system: uncoding pathogenesis of disease and illuminating components of immune control. Curr Top Microbiol Immunol. 2009; 330:31–54.
Article15. Griffin DE, Lin WH, Pan CH. Measles virus, immune control, and persistence. FEMS Microbiol Rev. 2012; 36:649–662.
Article16. Tucker WG, Andrew Paskauskas R. The MSMV hypothesis: measles virus and multiple sclerosis, etiology and treatment. Med Hypotheses. 2008; 71:682–689.
Article17. Zhu B, Guleria I, Khosroshahi A, Chitnis T, Imitola J, Azuma M, et al. Differential role of programmed deathligand 1 [corrected] and programmed death-ligand 2 [corrected] in regulating the susceptibility and chronic progression of experimental autoimmune encephalomyelitis. J Immunol. 2006; 176:3480–3489.
Article18. Salama AD, Chitnis T, Imitola J, Ansari MJ, Akiba H, Tushima F, et al. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med. 2003; 198:71–78.
Article19. Kroner A, Schwab N, Ip CW, Ortler S, Gobel K, Nave KA, et al. Accelerated course of experimental autoimmune encephalomyelitis in PD-1-deficient central nervous system myelin mutants. Am J Pathol. 2009; 174:2290–2299.
Article20. Ishizaki Y, Takemoto M, Kira R, Kusuhara K, Torisu H, Sakai Y, et al. Association of toll-like receptor 3 gene polymorphism with subacute sclerosing panencephalitis. J Neurovirol. 2008; 14:486–491.
Article21. Lafon M, Megret F, Meuth SG, Simon O, Velandia Romero ML, Lafage M, et al. Detrimental contribution of the immuno-inhibitor B7-H1 to rabies virus encephalitis. J Immunol. 2008; 180:7506–7515.
Article22. Mueller SN, Vanguri VK, Ha SJ, West EE, Keir ME, Glickman JN, et al. PD-L1 has distinct functions in hematopoietic and nonhematopoietic cells in regulating T cell responses during chronic infection in mice. J Clin Invest. 2010; 120:2508–2515.
Article23. Beutels P, Van Damme P, Van Casteren V, Gay NJ, De Schrijver K, Meheus A. The difficult quest for data on "vanishing" vaccine-preventable infections in Europe: the case of measles in Flanders (Belgium). Vaccine. 2002; 20:3551–3559.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Development of NKT Cells in Thymus is Defective in CD45 Knockout Mice
- Two Cases of Vaccine-Associated Measles in Daegu, South Korea, 2019
- Modified Measles in an Anti-Measles Immunoglobulin G-negative Healthcare Worker who had Received Two Doses of Measles-Containing Vaccine
- Difference in Resistance to Streptococcus pneumoniae Infection in Mice
- Expression of Aquaporin-3 and Aquaporin-4 in Aquaporin-1 Knockout Mice