Clin Exp Otorhinolaryngol.
2009 Sep;2(3):136-140.
Early Compliance and Efficacy of Sublingual Immunotherapy in Patients with Allergic Rhinitis for House Dust Mites
- Affiliations
-
- 1Department of Otorhinolaryngology, Seoul National University College of Medicine, Seoul National University, Seoul, Korea. csrhee@snu.ac.kr
- 2Institute of Allergy and Clinical Immunology, Medical Research Center, Seoul National University, Seoul, Korea.
- 3Sensory Organ Research Institute, Medical Research Center, Seoul National University, Seoul, Korea.
Abstract
OBJECTIVES
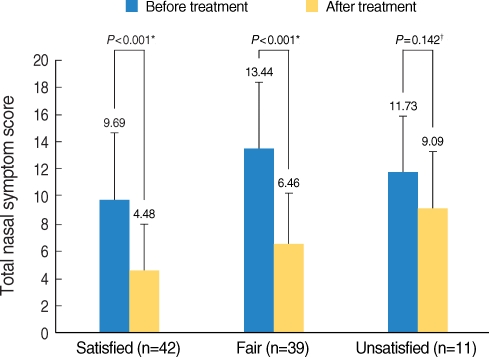
Sublingual immunotherapy (SLIT) has recently received much attention around the world as a treatment for allergic rhinitis. This study aimed to investigate the efficacy and adverse effects of SLIT in Korean patients with allergic rhinitis caused by house dust mites. The treatment compliance and the patient satisfaction with SLIT were also assessed. METHODS: The patients who were sensitized to Dermatophagoides pteronyssinus and Dermatophagoides farinae and who started SLIT between November 2007 and July 2008 were included in this study. The symptom questionnaires, which included items on rhinorrhea, sneezing, nasal obstruction, itchy nose, olfactory disturbance, eye discomfort and sleep disturbance, were obtained before and 6 months after SLIT. The patient satisfaction and the adverse effects were also investigated. RESULTS: One hundred forty-two patients started SLIT and 98 of them continued SLIT for 6 months or more. Ninety-two of the 98 patients completed the questionnaires. The duration of receiving SLIT was 9.8 months on average (range, 6 to 13 months). All the symptoms of allergic rhinitis were improved with SLIT. Forty-five percent of the patients were satisfied for SLIT, while 12% were unsatisfied. The incidence of adverse effects was 12% during maintenance therapy, although it was 48% during the up-dosing phase. The drop-out rate of SLIT was 31.0%. CONCLUSION: The subjective symptoms were improved with SLIT in Korean patients with allergic rhinitis for house dust mites. Yet the drop out rate was high despite of the symptomatic improvement.
Keyword
MeSH Terms
Figure
Reference
-
1. Esch RE. Sublingual immunotherapy. Curr Opin Otolaryngol Head Neck Surg. 2008; 6. 16(3):260–264. PMID: 18475082.
Article2. Guez S, Vatrinet C, Fadel R, Andre C. House-dust-mite sublingual-swallow immunotherapy (SLIT) in perennial rhinitis: a double-blind, placebo-controlled study. Allergy. 2000; 4. 55(4):369–375. PMID: 10782522.
Article3. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008; 4. 63(Suppl 86):8–160. PMID: 18331513.4. Wilson DR, Torres LI, Durham SR. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2003; (2):CD002893. PMID: 12804442.
Article5. Casanovas M, Guerra F, Moreno C, Miguel R, Maranon F, Daza JC. Double-blind, placebo-controlled clinical trial of preseasonal treatment with allergenic extracts of Olea europaea pollen administered sublingually. J Investig Allergol Clin Immunol. 1994; Nov–Dec. 4(6):305–314.6. Durham SR, Ying S, Varney VA, Jacobson MR, Sudderick RM, Mackay IS, et al. Grass pollen immunotherapy inhibits allergen-induced infiltration of CD4+ T lymphocytes and eosinophils in the nasal mucosa and increases the number of cells expressing messenger RNA for interferon-gamma. J Allergy Clin Immunol. 1996; 6. 97(6):1356–1365. PMID: 8648033.7. Pradalier A, Basset D, Claudel A, Couturier P, Wessel F, Galvain S, et al. Sublingual-swallow immunotherapy (SLIT) with a standardized five-grass-pollen extract (drops and sublingual tablets) versus placebo in seasonal rhinitis. Allergy. 1999; 8. 54(8):819–828. PMID: 10485385.
Article8. Purello-D'Ambrosio F, Gangemi S, Isola S, La Motta N, Puccinelli P, Parmiani S, et al. Sublingual immunotherapy: a double-blind, placebo-controlled trial with Parietaria judaica extract standardized in mass units in patients with rhinoconjunctivitis, asthma, or both. Allergy. 1999; 9. 54(9):968–973. PMID: 10505460.9. Dahl R, Kapp A, Colombo G, de Monchy JG, Rak S, Emminger W, et al. Efficacy and safety of sublingual immunotherapy with grass allergen tablets for seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006; 8. 118(2):434–440. PMID: 16890769.
Article10. Durham SR, Yang WH, Pedersen MR, Johansen N, Rak S. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006; 4. 117(4):802–809. PMID: 16630937.
Article11. Nuhoglu Y, Ozumut SS, Ozdemir C, Ozdemir M, Nuhoglu C, Erguven M. Sublingual immunotherapy to house dust mite in pediatric patients with allergic rhinitis and asthma: a retrospective analysis of clinical course over a 3-year follow-up period. J Investig Allergol Clin Immunol. 2007; 17(6):375–378.12. Passalacqua G, Guerra L, Pasquali M, Canonica GW. Non-injection routes for allergen immunotherapy: focus on sublingual immunotherapy. Inflamm Allergy Drug Targets. 2006; 1. 5(1):43–51. PMID: 16613563.
Article13. Kim DY, Kwon BW, Son JY. Assessment of satisfaction in patients undergoing immunotherapy for allergic rhinitis using questionnaires. Korean J Otolaryngol-Head Neck Surg. 004; 2. 47(2):132–138.14. Frew AJ. Sublingual immunotherapy. N Engl J Med. 2008; 5. 358(21):2259–2264. PMID: 18499568.
Article15. Antico A, Pagani M, Crema A. Anaphylaxis by latex sublingual immunotherapy. Allergy. 2006; 10. 61(10):1236–1237. PMID: 16942577.
Article16. Dunsky EH, Goldstein MF, Dvorin DJ, Belecanech GA. Anaphylaxis to sublingual immunotherapy. Allergy. 2006; 10. 61(10):1235. PMID: 16942576.
Article17. Eifan AO, Keles S, Bahceciler NN, Barlan IB. Anaphylaxis to multiple pollen allergen sublingual immunotherapy. Allergy. 2007; 5. 62(5):567–568. PMID: 17313400.
Article18. Canonica GW, Passalacqua G. Sublingual immunotherapy in the treatment of adult allergic rhinitis patients. Allergy. 2006; 61(Suppl 81):20–23. PMID: 16792602.
Article19. Lombardi C, Gargioni S, Melchiorre A, Tiri A, Falagiani P, Canonica GW, et al. Safety of sublingual immunotherapy with monomeric allergoid in adults: multicenter post-marketing surveillance study. Allergy. 2001; 10. 56(10):989–992. PMID: 11576079.
Article20. Rienzo VD, Minelli M, Musarra A, Sambugaro R, Pecora S, Canonica WG, et al. Post-marketing survey on the safety of sublingual immunotherapy in children below the age of 5 years. Clin Exp Allergy. 2005; 5. 35(5):560–564. PMID: 15898975.
Article21. Roder E, Berger MY, de Groot H, Gerth van Wijk R. Sublingual immunotherapy in youngsters: adherence in a randomized clinical trial. Clin Exp Allergy. 2008; 10. 38(10):1659–1667. PMID: 18631346.22. Passalacqua G, Musarra A, Pecora S, Amoroso S, Antonicelli L, Cadario G, et al. Quantitative assessment of the compliance with a once-daily sublingual immunotherapy regimen in real life (EASY Project: Evaluation of A novel SLIT formulation during a Year). J Allergy Clin Immunol. 2006; 4. 117(4):946–948. PMID: 16630956.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Outcome of Sublingual Immunotherapy in Patients With Allergic Rhinitis Sensitive to House Dust Mites
- Staloral(R) in Adult Patients with Allergic Rhinitis
- Update of Sublingual Immunotherapy for Allergic Rhinitis
- Distribution of House Dust Mites in the Bedroom of Patients with Allergic Rhinitis in Pusan Area
- Three-Year Follow-up Results of Sublingual Immunotherapy in Patients With Allergic Rhinitis Sensitized to House Dust Mites