Clin Exp Otorhinolaryngol.
2014 Mar;7(1):1-6.
Expression Profile of Fas-Fas Ligand in Spiral Ganglion Cells During Apoptosis
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Medical Science Research Institute, Dong-A University College of Medicine, Busan, Korea. doncamel@dau.ac.kr
- 2Department of Physiology, Medical Science Research Institute, Dong-A University College of Medicine, Busan, Korea.
Abstract
OBJECTIVES
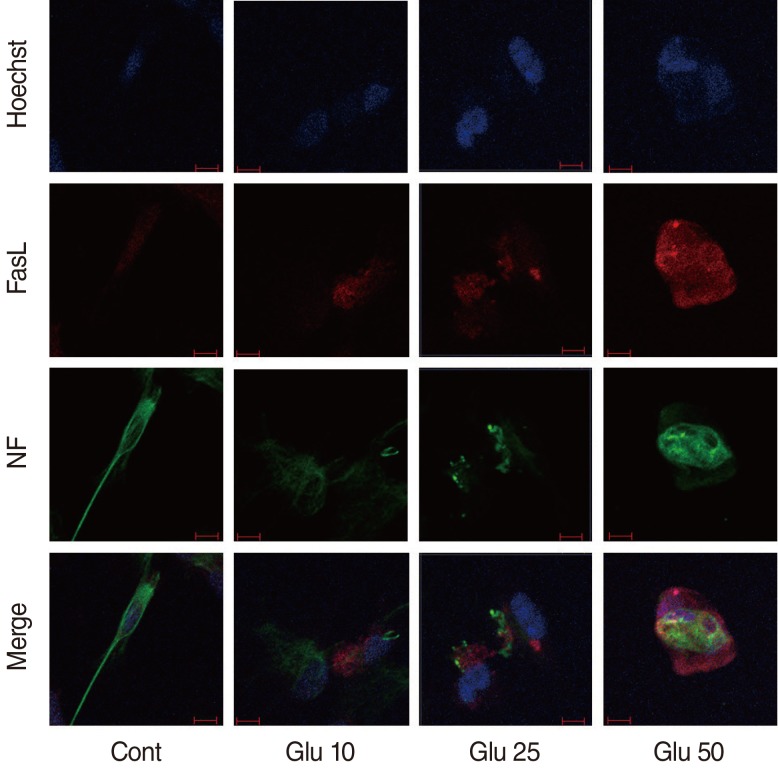
To examine the expression profile of Fas-Fas ligand (FasL) during glutamate (Glu)-induced spiral ganglion cell (SGC) apoptosis.
METHODS
Cultured SGCs were treated with 10-mM, 25-mM, and 50-mM concentrations of Glu and incubated for 24 or 48 hours. The expression intensity of FasL, Fas, caspase 3, and morphology of single SGC were evaluated using immunofluorescence staining.
RESULTS
In semiquantitative analysis of the Glu-treated SGC, FasL, and caspase 3 expression intensity were increased with concentration- and time-dependent manner. Fas expression intensity did not change with different concentration at 48 hours. In morphologic analysis of the Glu-treated SGC, number of apoptotic cells were increased with concentration- and time-dependent manner.
CONCLUSION
FasL was expressed in apoptotic SGCs, suggesting that the Fas-FasL signaling pathway may be involved in the Glu-induced apoptosis of dissociated SGCs.
Keyword
MeSH Terms
Figure
Reference
-
1. Puel JL. Chemical synaptic transmission in the cochlea. Prog Neurobiol. 1995; 12. 47(6):449–476. PMID: 8787031.
Article2. Pujol R, Puel JL, Gervais d'Aldin C, Eybalin M. Pathophysiology of the glutamatergic synapses in the cochlea. Acta Otolaryngol. 1993; 5. 113(3):330–334. PMID: 8100108.
Article3. Janssen R, Schweitzer L, Jensen KF. Glutamate neurotoxicity in the developing rat cochlea: physiological and morphological approaches. Brain Res. 1991; 6. 552(2):255–264. PMID: 1680530.
Article4. Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000; 10. 407(6805):802–809. PMID: 11048732.
Article5. Bae WY, Kim LS, Hur DY, Jeong SW, Kim JR. Secondary apoptosis of spiral ganglion cells induced by aminoglycoside: Fas-Fas ligand signaling pathway. Laryngoscope. 2008; 9. 118(9):1659–1668. PMID: 18758324.
Article6. Lee HK, Shin YK, Jung J, Seo SY, Baek SY, Park HT. Proteasome inhibition suppresses Schwann cell dedifferentiation in vitro and in vivo. Glia. 2009; 12. 57(16):1825–1834. PMID: 19455715.7. Lee HK, Seo IA, Suh DJ, Hong JI, Yoo YH, Park HT. Interleukin-6 is required for the early induction of glial fibrillary acidic protein in Schwann cells during Wallerian degeneration. J Neurochem. 2009; 2. 108(3):776–786. PMID: 19187095.
Article8. Ylikoski J, Wersall J, Bjorkroth B. Degeneration of neural elements in the cochlea of the guinea-pig after damage to the organ of corti by ototoxic antibiotics. Acta Otolaryngol Suppl. 1974; 326:23–41. PMID: 4534030.
Article9. Lefebvre PP, Weber T, Rigo JM, Staecker H, Moonen G, Van De Water TR. Peripheral and central target-derived trophic factor(s) effects on auditory neurons. Hear Res. 1992; 3. 58(2):185–192. PMID: 1568940.
Article10. Kopke R, Staecker H, Lefebvre P, Malgrange B, Moonen G, Ruben RJ, et al. Effect of neurotrophic factors on the inner ear: clinical implications. Acta Otolaryngol. 1996; 3. 116(2):248–252. PMID: 8725525.
Article11. Staecker H, Kopke R, Malgrange B, Lefebvre P, Van de Water TR. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport. 1996; 3. 7(4):889–894. PMID: 8724667.
Article12. Krieglstein K, Strelau J, Schober A, Sullivan A, Unsicker K. TGF-beta and the regulation of neuron survival and death. J Physiol Paris. 2002; Jan-Mar. 96(1-2):25–30. PMID: 11755780.13. Marzella PL, Gillespie LN, Clark GM, Bartlett PF, Kilpatrick TJ. The neurotrophins act synergistically with LIF and members of the TGF-beta superfamily to promote the survival of spiral ganglia neurons in vitro. Hear Res. 1999; 12. 138(1-2):73–80. PMID: 10575116.14. Ruel J, Chen C, Pujol R, Bobbin RP, Puel JL. AMPA-preferring glutamate receptors in cochlear physiology of adult guinea-pig. J Physiol. 1999; 8. 518(Pt 3):667–680. PMID: 10420005.
Article15. Jeong SW, Kim LS, Hur D, Bae WY, Kim JR, Lee JH. Gentamicin-induced spiral ganglion cell death: apoptosis mediated by ROS and the JNK signaling pathway. Acta Otolaryngol. 2010; 6. 130(6):670–678. PMID: 20082569.
Article16. Lee JE, Nakagawa T, Kim TS, Iguchi F, Endo T, Dong Y, et al. A novel model for rapid induction of apoptosis in spiral ganglions of mice. Laryngoscope. 2003; 6. 113(6):994–999. PMID: 12782811.
Article17. Alam SA, Ikeda K, Oshima T, Suzuki M, Kawase T, Kikuchi T, et al. Cisplatin-induced apoptotic cell death in Mongolian gerbil cochlea. Hear Res. 2000; 3. 141(1-2):28–38. PMID: 10713493.
Article18. Alam SA, Oshima T, Suzuki M, Kawase T, Takasaka T, Ikeda K. The expression of apoptosis-related proteins in the aged cochlea of Mongolian gerbils. Laryngoscope. 2001; 3. 111(3):528–534. PMID: 11224787.
Article19. Watanabe K, Tomiyama S, Jinnouchi K, Yagi T. Expression of caspase-activated deoxyribonuclease (CAD) and caspase 3 (CPP32) in the hydropic cochlea of guinea pigs: second report. Eur Arch Otorhinolaryngol. 2002; 5. 259(5):257–261. PMID: 12107529.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Fas/FasL in Radiation Induced Apoptosis in vivo
- The Expression of Fas Ligand protein in Keratoconus
- Effects of Cycloheximide and Dexamethasone on Fas - Mediated Apopthsis in Primary Human Astrocytes
- Expression of Fas and Fas Ligand in Various Skin Diseases
- Maternal serum soluble Fas/Fas ligand level and expression of Fas/Fas ligand in placenta in preeclampsia