Clin Exp Otorhinolaryngol.
2014 Dec;7(4):269-274. 10.3342/ceo.2014.7.4.269.
The Expression of AGO2 and DGCR8 in Idiopathic Sudden Sensorineural Hearing Loss
- Affiliations
-
- 1Department of Otolaryngology, Keimyung University School of Medicine, Daegu, Korea. entnamsi@dsmc.or.kr
- 2Department of Immunology, Keimyung University School of Medicine, Daegu, Korea.
- 3Department of Preventive Medicine, Keimyung University School of Medicine, Daegu, Korea.
- KMID: 2278366
- DOI: http://doi.org/10.3342/ceo.2014.7.4.269
Abstract
OBJECTIVES
The microRNAs have been implicated in the development and function of the inner ear, especially in contribution to hearing. However, the impact of idiopathic sudden sensorineural hearing loss (SSNHL) on expression of miRNA biogenesis-related components has not been established. To investigate the regulations of microRNA (miRNA) biogenesis-related components, argonaute 2 (AGO2) and DiGeorge syndrome critical region gene 8 (DGCR8) mRNA expression in SSNHL and to evaluate the value of clinical parameters on their expression.
METHODS
Thirty-seven patients diagnosed with SSNHL and fifty-one healthy volunteers were included in this study. We measured mRNA expression levels of AGO2 and DGCR8 in whole blood cells but erythrocytes of patients with SSNHL and controls, using reverse transcription and real-time polymerase chain reaction analysis.
RESULTS
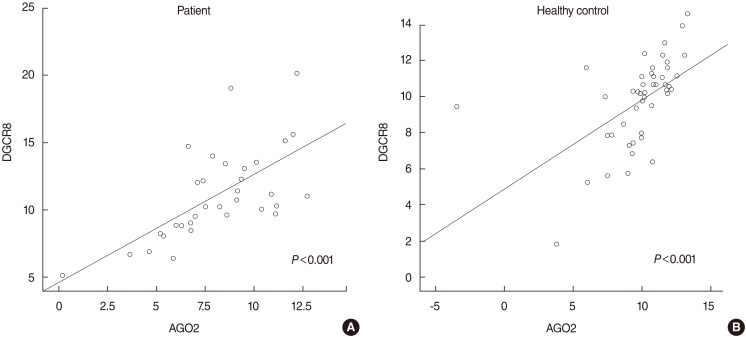
The mRNA expression level of AGO2 is upregulated in SSNHL. The expression level of AGO2 was significantly correlated with that of DGCR8 in both patients with SSNHL and controls. Expression level of AGO2 in SSNHL was correlated with white blood cell counts.
CONCLUSION
This study demonstrated for the first time that the AGO2 mRNA expression level was upregulated in SSNHL, suggesting its important role in pathobiology of SSNHL development.
Keyword
MeSH Terms
Figure
Reference
-
1. Stachler RJ, Chandrasekhar SS, Archer SM, Rosenfeld RM, Schwartz SR, Barrs DM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2012; 3. 146(3 Suppl):S1–S35. PMID: 22383545.
Article2. Byl FM Jr. Sudden hearing loss: eight years' experience and suggested prognostic table. Laryngoscope. 1984; 5. 94(5 Pt 1):647–661. PMID: 6325838.3. Zadeh MH, Storper IS, Spitzer JB. Diagnosis and treatment of sudden-onset sensorineural hearing loss: a study of 51 patients. Otolaryngol Head Neck Surg. 2003; 1. 128(1):92–98. PMID: 12574765.
Article4. Yossepowitch O, Lossos A, Lossos IS. Sudden hearing loss following acute hepatitis. Postgrad Med J. 1999; 5. 75(883):309–312. PMID: 10533644.
Article5. Suckfull M. Perspectives on the pathophysiology and treatment of sudden idiopathic sensorineural hearing loss. Dtsch Arztebl Int. 2009; 10. 106(41):669–675. PMID: 19946432.6. Nam SI, Yu GI, Kim HJ, Park KO, Chung JH, Ha E, et al. A polymorphism at -1607 2G in the matrix metalloproteinase-1 (MMP-1) increased risk of sudden deafness in Korean population but not at -519A/G in MMP-1. Laryngoscope. 2011; 1. 121(1):171–175. PMID: 21154774.
Article7. Nam SI, Ha E, Jung KH, Baik HH, Yoon SH, Park HJ, et al. IL4 receptor polymorphism is associated with increased risk of sudden deafness in Korean population. Life Sci. 2006; 1. 78(6):664–667. PMID: 16280132.8. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004; 1. 116(2):281–297. PMID: 14744438.9. Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, et al. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004; 11. 432(7014):235–240. PMID: 15531877.
Article10. Sand M, Skrygan M, Georgas D, Arenz C, Gambichler T, Sand D, et al. Expression levels of the microRNA maturing microprocessor complex component DGCR8 and the RNA-induced silencing complex (RISC) components argonaute-1, argonaute-2, PACT, TARBP1, and TARBP2 in epithelial skin cancer. Mol Carcinog. 2012; 11. 51(11):916–922. PMID: 22025453.
Article11. Groves AK, Zhang KD, Fekete DM. The genetics of hair cell development and regeneration. Annu Rev Neurosci. 2013; 7. 36:361–381. PMID: 23724999.
Article12. Zhang Q, Liu H, McGee J, Walsh EJ, Soukup GA, He DZ. Identifying microRNAs involved in degeneration of the organ of corti during age-related hearing loss. PLoS One. 2013; 4. 8(4):e62786. PMID: 23646144.
Article13. Inchley CS, Sonerud T, Fjærli HO, Nakstad B. Reduced Dicer expression in the cord blood of infants admitted with severe respiratory syncytial virus disease. BMC Infect Dis. 2011; 3. 11:59. PMID: 21385408.
Article14. Haussecker D, Huang Y, Lau A, Parameswaran P, Fire AZ, Kay MA. Human tRNA-derived small RNAs in the global regulation of RNA silencing. RNA. 2010; 4. 16(4):673–695. PMID: 20181738.
Article15. Smith KM, Guerau-de-Arellano M, Costinean S, Williams JL, Bottoni A, Mavrikis Cox G, et al. miR-29ab1 deficiency identifies a negative feedback loop controlling Th1 bias that is dysregulated in multiple sclerosis. J Immunol. 2012; 8. 189(4):1567–1576. PMID: 22772450.
Article16. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008; 3(6):1101–1108. PMID: 18546601.
Article17. Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998; 2. 391(6669):806–811. PMID: 9486653.
Article18. Kim B, Lee JH, Park JW, Kwon TK, Baek SK, Hwang I, et al. An essential microRNA maturing microprocessor complex component DGCR8 is up-regulated in colorectal carcinomas. Clin Exp Med. 2014; 8. 14(3):331–336. PMID: 23775303.
Article19. Zhang YN, Cao PP, Zhang XH, Lu X, Liu Z. Expression of microRNA machinery proteins in different types of chronic rhinosinusitis. Laryngoscope. 2012; 12. 122(12):2621–2627. PMID: 22961479.
Article20. Mockenhaupt S, Schurmann N, Grimm D. When cellular networks run out of control: global dysregulation of the RNAi machinery in human pathology and therapy. Prog Mol Biol Transl Sci. 2011; 102:165–242. PMID: 21846572.21. Jaffe BF. Clinical studies in sudden deafness. Adv Otorhinolaryngol. 1973; 20:221–228. PMID: 4351035.
Article22. Saeki N, Kitahara M. Assessment of prognosis in sudden deafness. Acta Otolaryngol Suppl. 1994; 510:56–61. PMID: 8128875.
Article23. Enache R, Sarafoleanu C. Prognostic factors in sudden hearing loss. J Med Life. 2008; Jul-Sep. 1(3):343–347. PMID: 20108511.24. Wei X, He J. Analysis of prognostic factors for sudden sensorineural hearing loss. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011; 7. 25(13):599–601. PMID: 21949992.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dicer Is Down-regulated and Correlated with Drosha in Idiopathic Sudden Sensorineural Hearing Loss
- Clinical Application of Hyperbaric Oxygen in Treatment of Idiopathic Sudden Sensorineural Hearing Loss
- Stellate Ganglion Block in Pediatric Patient with Idiopathic Sudden Sensorineural Hearing Loss : A case report
- Hyperbaric Oxygen Therapy for Sudden Sensorineural Hearing Loss after Failure from Oral and Intratympanic Corticosteroid
- The Characteristics and the Changes of Tinnitus according to the Recovery of Hearing Loss in the Patients with Sudden Hearing Loss