Korean J Obstet Gynecol.
2010 Jul;53(7):616-625. 10.5468/kjog.2010.53.7.616.
Inhibition of cell growth and apoptosis in CaSki, cervical cancer cell line by arsenic compounds
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Inje University College of Medicine, Busan Paik Hospital, Busan, Korea. buzzmi@chol.com
- 2Paik Institute for Clinical Research, Inje University College of Medicine, Busan Paik Hospital, Busan, Korea.
- KMID: 2273942
- DOI: http://doi.org/10.5468/kjog.2010.53.7.616
Abstract
OBJECTIVE
To compare inhibition of cell growth and apoptosis in human cervical cancer cell lines (CaSki) by paclitaxel, cisplatin, arsenic trioxide and tetraarsenic oxide.
METHODS
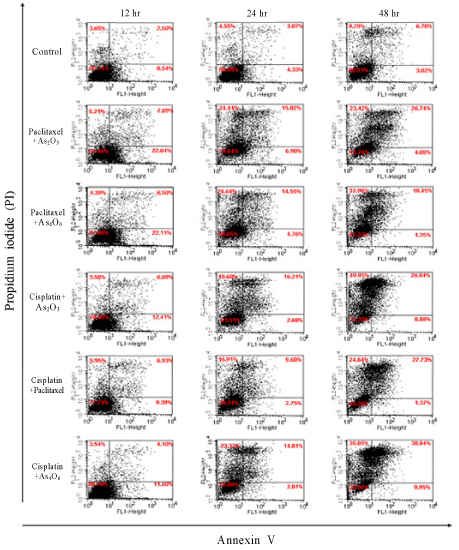
Inhibition of cell growth was determined by the water-soluble tetrazolium salts (WSTs) -1 assay. For apoptosis analysis in CaSki cell line treated with single or combination of two agents, CaSki cell line treated with each agent was stained with annexin-V/PI and flow cytometry was performed.
RESULTS
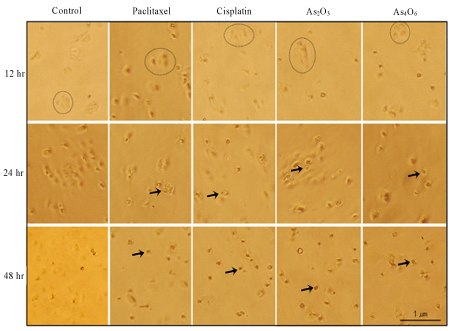
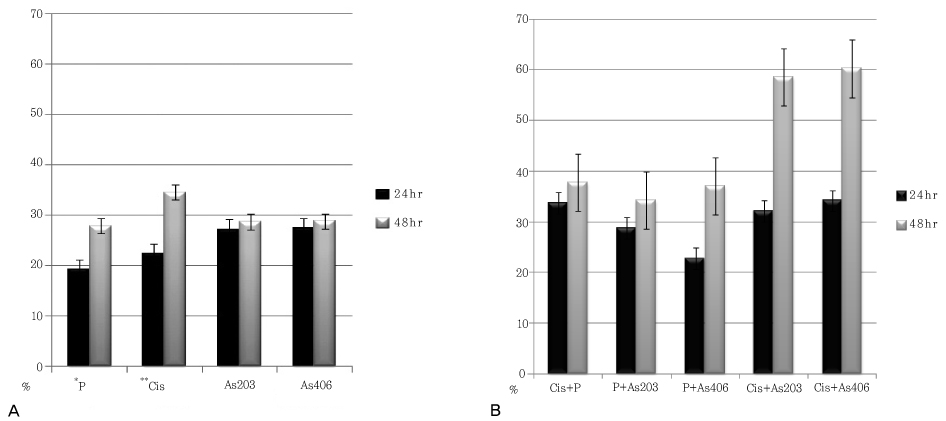
Progression of apoptosis in CaSki cell line treated with paclitaxel, cisplatin, arsenic trioxide, and tetraarsenic oxide was time dependent. Inhibition of cell growth in CaSki cell line by paclitaxel, cisplatin, arsenic trioxide, and tetraarsenic oxide was dose and time dependent. Especially, tetraarsenic oxide was more effective in inhibition of CaSki cell growth compared to arsenic trioxide. Group treated with combination of cisplatin and tetraarsenic oxide showed more progressive apoptosis compared to other combination group.
CONCLUSION
Tetraarsenic oxide has more potent anti-tumor effects than other agents on CaSki cell line. We need to consider further study about antitumor effect of tetraarsenic oxide through clinical study.
MeSH Terms
Figure
Reference
-
1. Garcia M, Jemal A, Ward EM, Center MM, Hao Y, Siegel RL, Thun MJ. Global Cancer Facts & Figures 2007 [Internet]. 2007. cited 2008 Jan 26. Atlanta, GA: American Cancer Society;Available from: URL: http://www.cancer.org/downloads/STT/Global_Cancer_Facts_and_Figures_2007_rev.pdf[about 3p. Figure 3].2. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009. 59:225–249.3. Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002. 347:1645–1651.4. Lee HP. Annual report of Gynecologic Cancer Registry Program in Korean for 2002 (Jan. 1st, 2002 - Dec. 31st, 2002). Korean J Obstet Gynecol. 2007. 50:28–78.5. Tao X, Hu W, Ramirez PT, Kavanagh JJ. Chemotherapy for recurrent and metastatic cervical cancer. Gynecol Oncol. 2008. 110:S67–S71.6. Thigpen T. The role of chemotherapy in the management of carcinoma of the cervix. Cancer J. 2003. 9:425–432.7. Long HJ 3rd. Management of metastatic cervical cancer: review of the literature. J Clin Oncol. 2007. 25:2966–2974.8. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995. 1:27–31.9. Sun HD, Ma L, Hu XC, Zhang TD. Treatment of acute promyelocytic leukemia by Ailing-1 therapy with use of syndrome differentiation of traditional Chinese medicine. Chin J Comb Trad Chin Med West Med. 1992. 12:170–171.10. Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998. 339:1341–1348.11. Wang ZY. Arsenic compounds as anticancer agents. Cancer Chemother Pharmacol. 2001. 48:Suppl 1. S72–S76.12. Akao Y, Yamada H, Nakagawa Y. Arsenic-induced apoptosis in malignant cells in vitro. Leuk Lymphoma. 2000. 37:53–63.13. Park MJ, Park IC, Bae IJ, Seo KM, Lee SH, Hong SI, et al. Tetraarsenic oxide, a novel orally administrable angiogenesis inhibitor. Int J Oncol. 2003. 22:1271–1276.14. Woo SH, Park MJ, An S, Lee HC, Jin HO, Lee SJ, et al. Diarsenic and tetraarsenic oxide inhibit cell cycle progression and bFGF- and VEGF-induced proliferation of human endothelial cells. J Cell Biochem. 2005. 95:120–130.15. Yoo MH, Kim JT, Rhee CH, Park MJ, Bae IJ, Yi NY, et al. Reverse effects of tetraarsenic oxide on the angiogenesis induced by nerve growth factor in the rat cornea. J Vet Med Sci. 2004. 66:1091–1095.16. Park IC, Park MJ, Woo SH, Lee HC, An S, Gwak HS, et al. Tetraarsenic oxide induces apoptosis in U937 leukemic cells through a reactive oxygen species-dependent pathway. Int J Oncol. 2003. 23:943–948.17. Smith JA, Ngo H, Martin MC, Wolf JK. An evaluation of cytotoxicity of the taxane and platinum agents combination treatment in a panel of human ovarian carcinoma cell lines. Gynecol Oncol. 2005. 98:141–145.18. Huh CY, Lim MC, Suh BS. Study on measure of chemosensitivities to topotecan, cisplatin and taxol theraphy in ovarian cancer cell lines: relationship with p53 and bcl-2 expression and apoptosis. Korean J Obstet Gynecol. 2003. 46:1368–1377.19. Kim J, Bae SM, Lim DS, Kwak SY, Lee CK, Lee YS, et al. Retraction: tetraarsenic oxide-mediated apoptosis in a cervical cancer cell line, SiHa. 2007. 39:48.20. Bae SM, Eu JY, Lee YJ, An WS. Apoptosis-induced cell growth inhibitory effects of a novel compound, As4O6 in a cervical cancer cell line, siHa in vitro. J Korean Assoc Cancer Prev. 2006. 11:39–45.21. Horwitz SB, Cohen D, Rao S, Ringel I, Shen HJ, Yang CP. Taxol: mechanisms of action and resistance. J Natl Cancer Inst Monogr. 1993. 55–61.22. Wang TH, Wang HS, Soong YK. Paclitaxel-induced cell death: where the cell cycle and apoptosis come together. Cancer. 2000. 88:2619–2628.23. Fisher DE. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994. 78:539–542.24. Eastman A. Activation of programmed cell death by anticancer agents: cisplatin as a model system. Cancer Cells. 1990. 2:275–280.25. Li X, Ding X, Adrian TE. Arsenic trioxide inhibits proliferation and induces apoptosis in pancreatic cancer cells. Anticancer Res. 2002. 22:2205–2213.26. Park WH, Seol JG, Kim ES, Hyun JM, Jung CW, Lee CC, et al. Arsenic trioxide-mediated growth inhibition in MC/CAR myeloma cells via cell cycle arrest in association with induction of cyclin-dependent kinase inhibitor, p21, and apoptosis. Cancer Res. 2000. 60:3065–3071.27. Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997. 89:3354–3360.28. Bazarbachi A, El-Sabban ME, Nasr R, Quignon F, Awaraji C, Kersual J, et al. Arsenic trioxide and interferon-alpha synergize to induce cell cycle arrest and apoptosis in human T-cell lymphotropic virus type I-transformed cells. Blood. 1999. 93:278–283.29. Ma DC, Sun YH, Chang KZ, Ma XF, Huang SL, Bai YH, et al. Selective induction of apoptosis of NB4 cells from G2+M phase by sodium arsenite at lower doses. Eur J Haematol. 1998. 61:27–35.30. Chang HS, Bae SM, Kim YW, Kwak SY, Min HJ, Bae IJ, et al. Comparison of diarsenic oxide and tetraarsenic oxide on anticancer effects: relation to the apoptosis molecular pathway. Int J Oncol. 2007. 30:1129–1135.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Apoptosis and Cell Cycle Arrest with EGF, TGF- a and TGF- 8 in Cervical Cancer Cell Lines
- Combined Effect of Radiation and Tyrphostin AG 1478 in Human Cervical Cancer Cell Lines
- Apigenin-induced apoptosis in cervical cancer cell lines
- Growth inhibition by fusidic acid in cervical, thyroid, and breast carcinoma cell lines
- The Gene Expression Profile Using cDNA microarray after treatment Arsenic Compound (As2O3, As4O6) in SiHa Cell