Korean J Nutr.
2012 Oct;45(5):411-419. 10.4163/kjn.2012.45.5.411.
Effects of the purified extracts from Lycii Cortex Radicis and ginger on lipid statusand serum cytokine levels in rats fed high fat diet
- Affiliations
-
- 1Department of Food Science and Nutrition, Catholic University of Daegu, Daegu 712-702, Korea. shcho@cu.ac.kr
- KMID: 2268735
- DOI: http://doi.org/10.4163/kjn.2012.45.5.411
Abstract
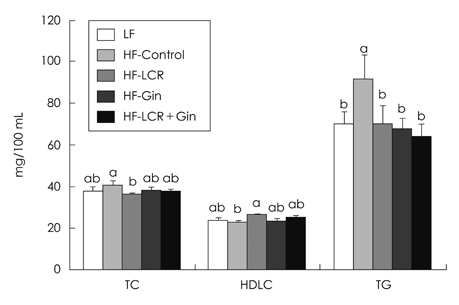
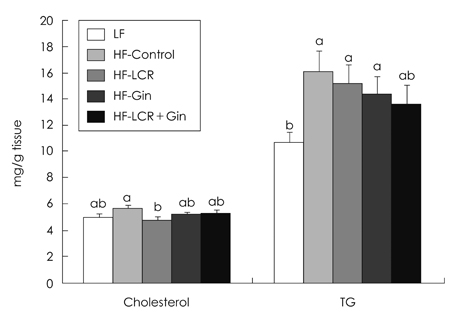
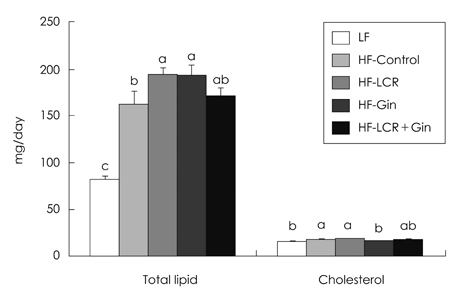
- The present study was to investigate the effects of Lycii Cortex Radicis (LCR), the root bark of lycium (Lycium chenese Miller) and ginger (Gin) on body lipid status and serum levels of cytokines. Sprague-Dawley (SD) male rats weighing 193.6 +/- 16.8 g were divided into five groups, including one low fat (LF) and four high fat groups, i.e. HF-Control, HF-LCR, HF-Gin and HF-LCR + Gin groups. Diets for HF-LCR, HF-Gin and HF-LCR + Gin groups contained purified extracts having 0.2 g LCR tyramine, ginerol and 0.1 g tyramine plus 0.02 g gingerol per kg, respectively. Compared with those of the HF-Control total serum cholesterol level decreased, and HDL-cholesterol level increased in the HF-LCR group and serum triglyceride levels decreased in the three experimental groups fed the purified extracts. Liver cholesterol level was lower in the HF-LCR group than the HF-Control group, but triglyceride levels, which were increased by high fat diets were not changed by significantly by LCR or ginger extracts. Fecal lipid excretion was higher in the HF-LCR and HF-Gin groups, but cholesterol excretion was lower in the HF-Gin group than in the HF-Control group. The activities of liver cytosolic glucose-6-phosphate dehydrogenase and malic enzyme were lower in the HF-LCR + Gin group than in the HF-Control group. Serum adiponectin levels did not differ among the five groups, while leptin level was lower in the HF-Gin group and C-reactive protein levels were lower in the HF-Gin and the HF-LCR + Gin groups than in the HF-Control group. It is concluded that LCR can be utilized as an ingredient for lipid-lowering functional foods in the form of purified extract and addition of small amount of ginger extract would be useful for reducing one of the inflammatory cytokines to help prevent atherosclerosis.
MeSH Terms
-
Adiponectin
Animals
Atherosclerosis
C-Reactive Protein
Catechols
Cholesterol
Cytokines
Cytosol
Diet
Diet, High-Fat
Fatty Alcohols
Functional Food
Ginger
Glucosephosphate Dehydrogenase
Humans
Leptin
Liver
Lycium
Male
Rats
Tyramine
Adiponectin
C-Reactive Protein
Catechols
Cholesterol
Cytokines
Fatty Alcohols
Glucosephosphate Dehydrogenase
Leptin
Tyramine
Figure
Reference
-
1. Potterat O. Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010. 76(1):7–19.
Article2. Yu MS, Lai CS, Ho YS, Zee SY, So KF, Yuen WH, Chang RC. Characterization of the effects of anti-aging medicine Fructus lycii on beta-amyloid peptide neurotoxicity. Int J Mol Med. 2007. 20(2):261–268.3. Kim OK. Antidiabetic and antioxidative effect of Lycii fructus in streptozotocin-induced diabetic rats. Korean J Pharmacogn. 2009. 40(2):128–136.4. Han SH, Park SH. Effect of Lycii fructus powder on lipid metabolism in 1% cholesterol fed rats. Korean J Food Cult. 2008. 23(4):521–528.5. Jeon YH, Moon JW, Kweon HJ, Jeoung YJ, An CS, Jin HL, Hur SJ, Lim BO. Effects of Lycii fructus and Astragalus membranaceus mixed extracts on immunomodulators and prevention of diabetic cataract and retinopathy in streptozotocin-induced diabetes rat model. Korean J Med Crop Sci. 2010. 18(1):15–21.6. Shon YG. Experimental studies of the effects of Lycii fructus, Lycii cortex radicis and Lycii folium on hypertension, hyperglycemia and hyperlipidemia [master's thesis]. 1993. Seoul: Kyung Hee University.7. Kim DH, Lee SY, Kim NK, Youn BK, Jung DS, Choi EY, Hong SR, Yoon JY, Kang MH, Lee JY. Moderating effects of skin hyperpigmentation from Lycii fructus and Lycii folium extracts. J Korean Soc Appl Biol Chem. 2011. 54(4):270–278.
Article8. Park JS, Seo GW, No JG, Cho IS, Park JH. Characteristics of the Gigolphy (Lycii cortex Radicis) wine. Korean J Med Crop Sci. 1995. 3(2):128–134.9. Morota T, Sasaki H, Chin M, Sato T, Katayama N, Fukuyama K, Mitsuhashi H. Studies on the crude drug containing the angiotensin I converting enzyme inhibitor (I). On the active principles of Lycium chinense Miller. Jpn J Pharmacogn. 1987. 41(3):169–173.10. Kim EO, Son WR, Kwon SO, Choi SW. Antilipidemic activity of phenolic compounds isolated from plant sources. In : Abstracts from Annual Meeting of Korean Society of Food Sci & Technology; 2010 June 16-18; Incheon. –247. Abstract No. p.11-75.11. Kim DJ, Kim JM, Kim TH, Baek JM, Kim HS, Choe M. Effects of mixed extract from Lycium chinense, Cordyceps militaris, and Acanthopanax senticosus on glucose-regulating enzymes of Hep-G2 in hyperglycemic conditions. J Korean Soc Food Sci Nutr. 2010. 39(9):1257–1262.
Article12. Gao D, Li Q, Liu Z, Li Y, Liu Z, Fan Y, Li K, Han Z, Li J. Hypoglycemic effects and mechanisms of action of Cortex Lycii Radicis on alloxan-induced diabetic mice. Yakugaku Zasshi. 2007. 127(10):1715–1721.
Article13. Ahn BY, Gwak JS, Ryu SH, Moon GS, Choi DS, Park SH, Han JH. Protective effect of water extract of Lycii Cordex Radicis on lipid peroxidation of rat skin exposed to ultraviolet B radiation. Agric Chem Biotechnol. 2002. 45(4):218–222.14. Kim SH, Cho YJ. Protective effect of EA fraction of Lycii Cortex Radix on the hepatic damage in mice induced by CCl4. Korean J Orient Med Pathol. 1997. 11(2):63–71.15. Cho SH, Park EJ, Kim EO, Choi SW. Study on the hypochlolesterolemic and antioxidative effects of tyramine derivatives from the root bark of Lycium chinense Miller. Nutr Res Pract. 2011. 5(5):412–420.
Article16. Connell DW, Sutherland MD. A re-examination of gingerol, shogaol, and zingerone, the pungent principles of ginger (Zingiber officinale Roscoe). Aust J Chem. 1969. 22(5):1033–1043.
Article17. Lee BS, Ko MS, Kim HJ, Kwak IS, Kim DH, Chung BW. Separation of 6-gingerol from ginger (Zingiber officinale Roscoe) and antioxidative activity. Korean J Biotechnol Bioeng. 2006. 21(6):484–488.18. Sheo HJ. The antibacterial action of garlic, onion, ginger and red pepper juice. J Korean Soc Food Sci Nutr. 1999. 28(1):94–99.19. Thomson M, Al-Qattan KK, Al-Sawan SM, Alnaqeeb MA, Khan I, Ali M. The use of ginger (Zingiber officinale Roscoe) as a potential anti-inflammatory and antithrombotic agent. Prostaglandins Leukot Essent Fatty Acids. 2002. 67(6):475–478.
Article20. Park KY, Lee SJ, Lee KI, Rhee SH. The antitumor effect in sarcoma-180 tumor cell of mice administered with Japanese apricot, garlic or ginger Doenjang. Korean J Food Cookery Sci. 2005. 21(5):599–606.21. Tejasari D. Evaluation of ginger (Zingiber officinale Roscoe) bioactive compounds in increasing the ratio of T-cell surface molecules of CD3+CD4+ : CD3+CD8+ in-vitro. Malays J Nutr. 2007. 13(2):161–170.22. Kim CJ. Atherosclerosis and inflammation. J Korean Soc Lipidol Atheroscler. 2001. 11(4):413–419.23. Park CY, Yoo HJ. Inflammation and obesity. J Korean Soc Endocrinol. 2004. 19(2):97–108.24. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993. 123(11):1939–1951.
Article25. Cho SH, Lee HR, Kim TH, Choi SW, Lee WJ, Choi Y. Effects of defatted safflower seed extract and phenolic compounds in diet on plasma and liver lipid in ovariectomized rats fed high-cholesterol diets. J Nutr Sci Vitaminol (Tokyo). 2004. 50(1):32–37.
Article26. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957. 226(1):497–509.
Article27. Omodeo Salè F, Marchesini S, Fishman PH, Berra B. A sensitive enzymatic assay for determination of cholesterol in lipid extracts. Anal Biochem. 1984. 142(2):347–350.
Article28. Bergmeyer HU. Bergmeyer HU, editor. Glucose-6-phosphate dehydrogenases. Methods of Enzymatic Analysis. 1974. 2nd English ed. New York: Academic Press;458–459.29. Geer BW, Krochko D, Oliver MJ, Walker VK, Williamson JH. A comparative study of the NADP-malic enzymes from Drosophila and chick liver. Comp Biochem Physiol B. 1980. 65(1):25–34.
Article30. Layne E. [73] Spectrophotometric and turbidimetric methods for measuring proteins. Methods Enzymol. 1957. 3:447–454.
Article31. Jeong TS, Choi MS, Park YB, Bok SH. Cholesterol-lowering or antiatherogenic effects of citrus bioflavonoids and their mechanisms. Food Ind Nutr. 2000. 5(2):21–26.32. Monforte MT, Trovato A, Kirjavainen S, Forestieri AM, Galati EM, Lo Curto RB. Biological effects of hesperidin, a Citrus flavonoid. (note II): hypolipidemic activity on experimental hypercholesterolemia in rat. Farmaco. 1995. 50(9):595–599.33. Suh SH, Lee HR, Rhee SJ, Choi SW, Cho SH. Effects of Peonia seed extracts and resveratrol on lipid metabolism in rats fed high cholesterol diets. J Korean Soc Food Sci Nutr. 2003. 32(7):1102–1107.
Article34. Kim YM, Han CK, Bang SJ, Park JH. Effects of laminaran from Eisenia bicyclis on serum lipids in rats fed high cholesterol diet. J Korean Soc Food Sci Nutr. 2006. 35(7):841–846.35. Kim HJ, Choi SW, Cho SH. Effects of various mulberry products on the blood glucose and lipid status of streptozotocin-induced diabetic rats. Korean J Nutr. 2010. 43(6):551–560.
Article36. Jeppesen J, Hein HO, Suadicani P, Gyntelberg F. Relation of high TG-low HDL cholesterol and LDL cholesterol to the incidence of ischemic heart disease. An 8-year follow-up in the Copenhagen Male Study. Arterioscler Thromb Vasc Biol. 1997. 17(6):1114–1120.
Article37. National Cholesterol Education Program, National Heart, Lung, and Blood Institute, National Institutes of Health. Third report of the expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). 2002. NIH Publication No. 02-5215.38. Shin JH, Lee SJ, Sung NJ. Effects of Zingiber mioga, Zingiber mioga root and Zingiber officinale on the lipid concentration in hyperlipidemic rats. J Korean Soc Food Sci Nutr. 2002. 31(4):679–684.39. Tepperman HM, Tepperman J. Patterns of dietary and hormonal induction of certain NADP-linked liver enzymes. Am J Physiol. 1964. 206:357–361.
Article40. Stumpo DJ, Kletzien RF. Regulation of glucose-6-phosphate dehydrogenase mRNA by insulin and the glucocorticoids in primary cultures of rat hepatocytes. Eur J Biochem. 1984. 144(3):497–502.
Article41. Suh M, Kim HM, Na HK, Cho SH. Effects of dietary n-3 fats on hepatic glucose-6-phosphate dehydrogenase and malic enzyme in rat. Korean Biochem J. 1990. 23(3):395–401.42. Zhang B, Xue C, Hu X, Xu J, Li Z, Wang J, Yanagita T, Xue Y, Wang Y. Dietary sea cucumber cerebroside alleviates orotic acid-induced excess hepatic adipopexis in rats. Lipids Health Dis. 2012. 11(1):48.
Article43. Ide T, Ono Y, Kawashima H, Kiso Y. Interrelated effects of dihomo-γ-linolenic and arachidonic acids, and sesamin on hepatic fatty acid synthesis and oxidation in rats. Br J Nutr. 2012. 1–14.
Article44. Suh J, Byun JD, On YK, Hyon MS, Kim SK, Kwon YJ. Oxidized LDL and inflammatory markers in ischemic heart disease. Korean J Med. 2003. 64(5):535–541.45. Dugasani S, Pichika MR, Nadarajah VD, Balijepalli MK, Tandra S, Korlakunta JN. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J Ethnopharmacol. 2010. 127(2):515–520.
Article46. Kim EY, Jung EY, Lim HS, Heo YR. The effects of the Sasa borealis leaves extract on plasma adiponectin, resistin, C-reactive protein and homocysteine levels in high fat diet-induced obese C57/BL6J mice. Korean J Nutr. 2007. 40(4):303–311.47. Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004. 24(1):29–33.
Article48. Havel PJ. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr Opin Lipidol. 2002. 13(1):51–59.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Liquid Culture of Coriolus Versicolor on Lipid Metabolism and Enzyme Activities in Rats Fed High Fat Diet
- Study on the hypochlolesterolemic and antioxidative effects of tyramine derivatives from the root bark of Lycium chenese Miller
- Effects of Liquid Culture of Agaricus blazei Murill on Lipid Metabolism and Enzyme Activities in Rats Fed High Fat Diet
- Effect of Chlorella vulgaris on lipid metabolism in Wistar rats fed high fat diet
- Effects of Genistein Supplementation on Fatty Liver and Lipid Metabolism in Rats Fed High Fat Diet