Nutr Res Pract.
2008 Dec;2(4):204-210. 10.4162/nrp.2008.2.4.204.
Effect of Chlorella vulgaris on lipid metabolism in Wistar rats fed high fat diet
- Affiliations
-
- 1Department of Nutritional Science and Food Management, Ewha Womans University, 11-1 Daehyeon-dong, Seodaemun-gu, Seoul 120-750, Korea. mkk@ewha.ac.kr
- KMID: 2139193
- DOI: http://doi.org/10.4162/nrp.2008.2.4.204
Abstract
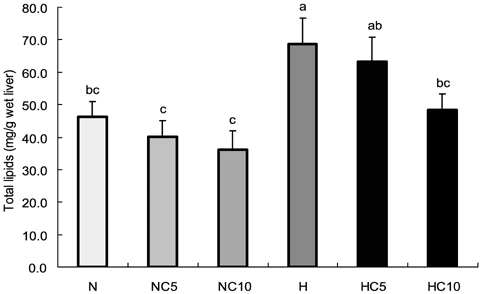
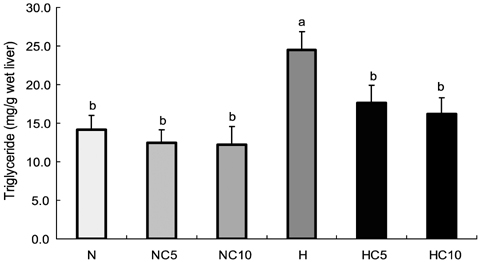
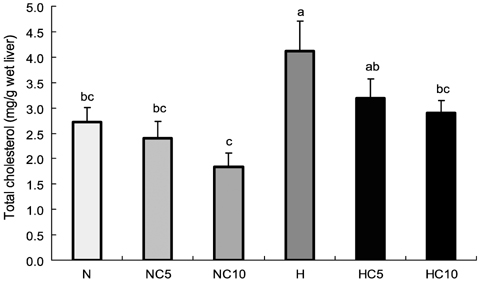
- This study was performed to investigate effects of Chlorella vulgaris on lipid metabolism in rats fed high fat diet. Sixty 6-week-old male Wistar rats were divided into two groups; normal diet group and high fat diet group, then the rats in each group were further divided into three subgroups and fed 0%, 5% and 10% (w/w) chlorella-containing diets, respectively, and raised for 9 weeks. Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activity and total protein and albumin concentration were not different among groups. Serum total lipids and liver TG concentration were significantly lower in 5% and 10% chlorella groups than 0% chlorella group in high fat diet groups (p<0.05). Serum TG, serum total cholesterol, liver total lipid and liver total cholesterol concentrations were significantly lower in 10% chlorella groups than 0% chlorella group in high fat diet groups (p<0.05). Fecal total lipid, TG and total cholesterol excretions were significantly higher in 5% and 10% chlorella groups than 0% chlorella groups in normal diet and high fat diet groups, respectively (p<0.05). These results suggest that Chlorella vulgaris is effective for prevention of dyslipidemia which may be due to the modulation of lipid metabolism and increased fecal excretion of lipid.
Keyword
MeSH Terms
Figure
Reference
-
1. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959. 37:911–917.
Article2. Boden G, Chen X, Iqbal N. Acute lowering of plasma fatty acids lowers basal insulin secretion in diabetic and nondiabetic subjects. Diabetes. 1998. 47:1609–1612.
Article3. Chau CF, Huang YL. Effects of the insoluble fiber derived from Passiflora edulis seed on plasma and hepatic lipids and fecal output. Mol Nutr Food Res. 2005. 49:786–790.
Article4. Cherng JY, Shih MF. Potential hypoglycemic effects of Chlorella in streptozo-tocin induced diabetic mice. Life Sci. 2005a. 77:980–990.5. Cherng JY, Shih MF. Preventing dyslipidemia by Chlorella pyrenoidosa in rats and hamsters after chronic high fat diet treatment. Life Sci. 2005b. 76:3001–3013.
Article6. Cherng JY, Shih MF. Improving glycogenesis in Streptozocin (STZ) diabetic mice after administration of green algae Chlorella. Life Sci. 2006. 78:1181–1186.
Article7. Frings CS, Dunn RT. A colorimetric method for determination of total serum lipid based on the sulfophospho-vanillin reaction. Am J Clin Pathol. 1970. 53:89–91.
Article8. Ghosh P, Bitsanis D, Ghebremeskel K, Crawford MA, Poston L. Abnormal aortic fatty acid composition and small artery function in offspring of rats fed a high fat diet in pregnancy. J Physiol. 2001. 533:815–822.
Article9. Han JG, Kang GG, Kim JK, Kim SH. The present status and future of chlorella. Food Science and Industry. 2002. 6:64–69.10. Huff MW, Carroll KK. Effects of dietary protein on turnover, oxidation and absorption of cholesterol, and on steroid excretion in rabbits. J Lipid Res. 1980. 21:546–558.
Article11. Iwami K, Sakakibara K, Ibuki F. Involvement of post-digestion hydrophobic peptides in plasma cholesterol-lowering effect of dietary plant protein. Agric Biol Chem. 1986. 50:1217–1222.
Article12. Kang MS, Sim AJ, Chae HJ. Chlorella as a Functional Biomaterial. Korean Journal of Biotechnology and Bioengineering. 2004. 19:1–11.13. Kay PA. Microalgae as food and supplement. Crit Rev Food Sci Nutr. 1991. 30:555–573.
Article14. Konishi F, Mitsuyama M, Okuda M, Tanaka K, Hasegawa H, Nomoto K. Protective effect of an acidic glycoprotein obtained from culture of Chlorella vulgaris against myelosuppression by 5-fluorouracil. Cancer Immunol Immunother. 1996. 42:268–274.
Article15. Kupeli E, Orhan DD, Yesilada E. Effect of Cistus laurifolius L. leaf extracts and flavonoids on acetaminophen induced hepatotoxicity in mice. J Ethnopharmacol. 2006. 103:455–460.16. Layne E. Spectrophotometric and turbidimetric methods for measuring proteins. Methods Enzymol. 1957. 3:447–454.17. Lee HS, Choi CY, Cho C, Song Y. Attenuating Effect of Chlorella Supplementation on Oxidative Stress and NF.KAPPA.B Activation in Peritoneal Macrophages and Liver of C57BL/6 Mice Fed on an Atherogenic Diet. Biosci Biotechnol Biochem. 2003. 67:2083–2090.
Article18. Lee SO, Simons AL, Murphy PA, Hendrich S. Soyasaponins lowered plasma cholesterol and increased fecal bile acids in female golden Syrian hamsters. Exp Biol Med. 2005. 230:472–478.
Article19. Nagata Y, Ishiwaki N, Sugano M. Studies on the mechanism of antihypercholesterolemic action of soy protein and soy protein-type amino acid mixtures in relation to the casein counterparts in rats. J Nutr Biochem. 1982. 112:1614–1625.
Article20. Okudo M, Hasegawa T, Sonoda M, Okabe T, Tanaka M. The effects of Chlorella on the level of cholesterol in serum and liver. Jap J Nutrition. 1975. 33:3–8.21. Park HS, Park JY, Cho HJ. Attitudes and reported practice for obesity management in Korea after introduction of anti-obesity agents. J Korean Med Sci. 2005. 20:1–6.
Article22. Queiroz ML, Bincoletto C, Valadares MC, Dantas DC, Santos LM. Effects of Chlorella vulgaris extract on cytokines production in Listeria monocytogenes infected mice. Immunopharmacol Immunotoxicol. 2002. 24:483–496.
Article23. Reaven GM, Chang H, Ho H, Jeng CY, Hoffman BB. Lowering of plasma glucose in diabetic rats by antilipolytic agents. Am J Physiol. 1988. 254:E23–E30.
Article24. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993. 123:1939–1951.
Article25. Reitman A, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957. 28:56–63.
Article26. Rizvi F, Iftikhar M, George JP. Beneficial effects of fish liver preparations of sea bass (Lates calcarifer) versus gemfibrozil in high fat diet-induced lipid-intolerant rats. J Med Food. 2003. 6:123–128.
Article27. Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996. 97:2859–2865.
Article28. Saloranta C, Franssila-Kallunki A, Ekstrand A, Taskinen MR, Groop L. Modulation of hepatic glucose production by non-esterified fatty acids in type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1991. 34:409–415.
Article29. Sano T, Tanaka Y. Effects of dried powdered Chlorella vulgaris on experimental atherosclerosis and alimentary hypercholesterolemia in cholesterol-fed rabbit. Artery. 1987. 14:760–784.30. Sano T, Kumamoto Y, Kamiya N, Okuda M, Tanaka Y. Effect of lipophilic extract of Chlorella vulgaris on alimentary hyperlipidemia in cholesterol-fed rats. Artery. 1988. 15:217–224.31. Shibata S, Oda K, Onodera-Masuoka N, Matsubara S, Kikuchi-Hayakawa H, Ishikawa F, Iwabuchi A, Sansawa H. Hypocholesterolemic effect of indigestible fraction of Chlorella regularis in cholesterol-fed rats. J Nutr Sci Vitaminol. 2001. 47:373–377.
Article32. Singh A, Singh SP, Bamazai R. Perinatal influence of Chlorella vulgaris on hepatic drug metabolizing enzyme and lipis. Anticancer Res. 1998. 18:1509–1514.33. Smith C, Marks AD, Lieberman M. . Basic medical biochemistry. 2005. Baltimore, USA: Lippincott Williams & Wilkins;478–479.34. Steinberg D. Antioxidants and atherosclerosis. A current assessment. 1991. 84:1240–1245.35. Storlien LH, Baur LA, Kriketos AD, Pan DA, Cooney GJ, Jenkins AB, Calvert GD, Campbell LV. Dietary fats and insulin action. Diabetologia. 1996. 39:621–631.
Article36. Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988. 37:1163–1167.
Article37. Tanaka K, Yamada A, Nada K, Shoyama Y, Kubo C, Nomoto K. Oral administration of a unicellular green algae, Chlorella vulgaris, prevents stress-induced ulcer. Plant Med. 1997. 63:465–466.
Article38. Tanaka K, Yamada A, Noda K, Hasegawa T, Okuda K, Shoyama Y, Nomoto K. A novel glycoprotein obtained from Chlorella vulgaris strain CK22 shows antimetastatic immunopotentiation. Cancer Immunol Immunother. 1998. 45:313–320.
Article39. The Koreas Society of Food Science and Nutrition. Handbook of experimental in food science and nutrition. 2000. Seoul, Republic of Korea: Hyoil Press;661.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hypoglycemic effect of Chlorella vulgaris intake in type 2 diabetic Goto-Kakizaki and normal Wistar rats
- Effect of Chlorella vulgaris intake on cadmium detoxification in rats fed cadmium
- Effects of Soyoligosaccharide on Lipid Metabolism in Rats Fed the High Fat or Low Fat Diet
- Effect of Dietary Grape Pomace on Lipid Oxidation and Related Enzyme Activities in Rats Fed High Fat Diet
- Effects of Liquid Culture of Coriolus Versicolor on Lipid Metabolism and Enzyme Activities in Rats Fed High Fat Diet