Ann Dermatol.
2013 Feb;25(1):17-22. 10.5021/ad.2013.25.1.17.
The Clinical Efficacy of Mometasone Furoate in Multi-Lamellar Emulsion for Eczema: A Double-blinded Crossover Study
- Affiliations
-
- 1Department of Dermatology, Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Incheon, Korea. drchosh@hotmail.com
- 2Department of Dermatology, Kangnam Sacred Heart Hospital, College of Medicine, Hallym University, Seoul, Korea.
- 3Department of Dermatology, College of Medicine, Seoul National University, Seoul, Korea.
- 4Department of Dermatology, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of Dermatology, Myongji Hospital, Kwandong University College of Medicine, Goyang, Korea.
- KMID: 2265966
- DOI: http://doi.org/10.5021/ad.2013.25.1.17
Abstract
- BACKGROUND
Topical application of corticosteroids also has an influence on skin barrier impairment. Physiological lipid mixtures, such as multi-lamellar emulsion (MLE) containing a natural lipid component leads to effective recovery of the barrier function.
OBJECTIVE
The purpose of this study was to conduct an evaluation of the therapeutic efficacy and skin barrier protection of topical mometasone furoate in MLE.
METHODS
A multi-center randomized, double-blind, controlled study was performed to assess the efficacy and safety of mometasone furoate cream in MLE for Korean patients with eczema. The study group included 175 patients with eczema, who applied either mometasone furoate in MLE cream or methylprednisolone aceponate cream for 2 weeks. Treatment efficacy was evaluated using the physician's global assessment of clinical response (PGA), trans-epidermal water loss (TEWL), and visual analogue scale (VAS) for pruritus. Patients were evaluated using these indices at days 4, 8, and 15.
RESULTS
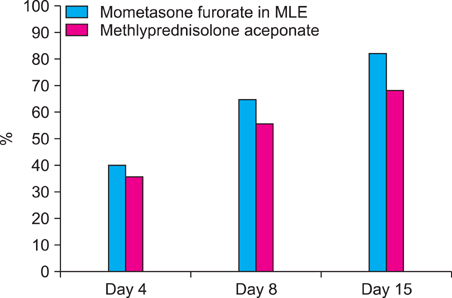
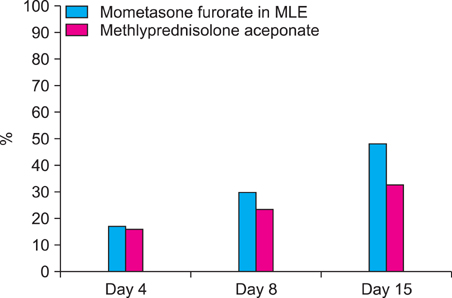
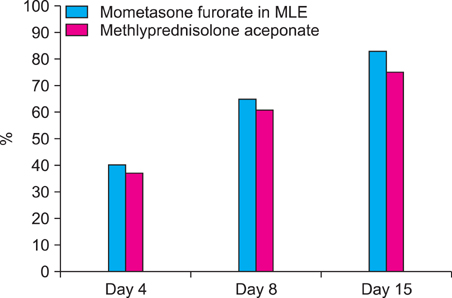
Comparison of PGA score, TEWL, and VAS score at baseline with those at days 4, 8, and 15 of treatment showed a significant improvement in both groups. Patients who applied mometasone furoate in MLE (74.8%) showed better results (p<0.05) than those who applied methylprednisolone aceponate (47.8%). The TEWL improvement ratio was higher in the mometasone furoate in MLE group than that in the methylprednisolone aceponate group, and VAS improvement was also better in the mometasone furoate in MLE group.
CONCLUSION
Mometasone furoate in MLE has a better therapeutic efficacy as well as less skin barrier impairment than methylprednisolone aceponate.
Keyword
MeSH Terms
Figure
Reference
-
1. Lange K, Gysler A, Bader M, Kleuser B, Korting HC, Schäfer-Korting M. Prednicarbate versus conventional topical glucocorticoids: pharmacodynamic characterization in vitro. Pharm Res. 1997. 14:1744–1749.2. Lubach D, Rath J, Kietzmann M. Steroid-induced dermal thinning: discontinuous application of clobetasol-17-propionate ointment. Dermatology. 1992. 185:44–48.
Article3. Leung DYM, Eichenfield LF, Boguniewicz M. Wolff K, Fitzpatrick TB, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, editors. Atopic dermatitis. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;146–158.4. Elias PM. Lipids and the epidermal permeability barrier. Arch Dermatol Res. 1981. 270:95–117.
Article5. Elias PM. Epidermal lipids, membranes, and keratinization. Int J Dermatol. 1981. 20:1–19.
Article6. Williams HC. Is the prevalence of atopic dermatitis increasing? Clin Exp Dermatol. 1992. 17:385–391.
Article7. Ogawa H, Yoshiike T. A speculative view of atopic dermatitis: barrier dysfunction in pathogenesis. J Dermatol Sci. 1993. 5:197–204.
Article8. Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Invest Dermatol. 1991. 96:523–526.
Article9. Lee EJ, Suhr KB, Lee JH, Park JK, Jin CY, Youm JK, et al. The clinical efficacy of a multi-lamellar emulsion containing pseudoceramide in childhood atopic dermatitis: an open crossover study. Ann Dermatol. 2003. 15:133–138.
Article10. Ahn SK, Bak HN, Park BD, Kim YH, Youm JK, Choi EH, et al. Effects of a multilamellar emulsion on glucocorticoid-induced epidermal atrophy and barrier impairment. J Dermatol. 2006. 33:80–90.
Article11. Chamlin SL, Frieden IJ, Fowler A, Williams M, Kao J, Sheu M, et al. Ceramide-dominant, barrier-repair lipids improve childhood atopic dermatitis. Arch Dermatol. 2001. 137:1110–1112.12. Hoybye S, Balk MS, De Cunha Bang F, Ottevanger V, Veien NK. Continuous and intermittent treatment of atopic dermatitis in adults with mometasone furoate versus hydrocortisone 17-butyrate. Curr Ther Res Clin Exp. 1991. 50:67–72.13. Rakja G, Avrach W, Gärtner L, Overgaard-Petersen H. Mometasone furoate 0.1% fatty cream once daily versus betamethasone valerate 0.1% cream twice daily in the treatment of patients with atopic and allergic contact dermatitis. Curr Ther Res. 1993. 54:23–29.
Article14. Faergemann J, Christensen O, Sjövall P, Johnsson A, Hersle K, Nordin P, et al. An open study of efficacy and safety of long-term treatment with mometasone furoate fatty cream in the treatment of adult patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2000. 14:393–396.
Article15. Man MQ M, Feingold KR, Thornfeldt CR, Elias PM. Optimization of physiological lipid mixtures for barrier repair. J Invest Dermatol. 1996. 106:1096–1101.
Article16. Kim HJ, Park HJ, Yun JN, Jeong SK, Ahn SK, Lee SH. Pseudoceramide-containing physiological lipid mixture reduces adverse effects of topical steroids. Allergy Asthma Immunol Res. 2011. 3:96–102.
Article17. Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006. 54:1–15.
Article18. Kang JS, Yoon WK, Youm JK, Jeong SK, Park BD, Han MH, et al. Inhibition of atopic dermatitis-like skin lesions by topical application of a novel ceramide derivative, K6PC-9p, in NC/Nga mice. Exp Dermatol. 2008. 17:958–964.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Topical Steroid Nasal Instillation in Induced Anosmic Mice
- The Clinical Efficacy of a Multi-Lamellar Emulsion Containing Pseudoceramide in Childhood Atopic Dermatitis: An Open Crossover Study
- In vivo and in vitro efficacy of florfenicol, terbinafine, and mometasone furoate topical otic solution for the treatment of canine otitis externa
- The Effect of Mometasone Furoate Cream on Skin Barrier Function in Patients with Allergic Contact Dermatitis
- Role of Intranasal Topical Steroid in Pediatric Sleep Disordered Breathing and Influence of Allergy, Sinusitis, and Obesity on Treatment Outcome