Clin Exp Otorhinolaryngol.
2011 Mar;4(1):27-32.
Role of Intranasal Topical Steroid in Pediatric Sleep Disordered Breathing and Influence of Allergy, Sinusitis, and Obesity on Treatment Outcome
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Samsung Changwon Hospital, Changwon, Korea.
- 2Department of Otorhinolaryngology-Head and Neck Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. hysiam.kim@samsung.com
Abstract
OBJECTIVES
To evaluate efficacy of short term intranasal corticosteroid (mometasone furoate) treatment in pediatric sleep-disordered breathing (SDB) patients.
METHODS
A prospective, observational study was done. A total of 41 children (2-11 years old) were enrolled into this study. All patients received 4-weeks course of mometasone furoate 100 microg/day treatment. They were evaluated at pretreatment and immediately after treatment with obstructive sleep apnea (OSA)-18 quality of life survey and lateral neck X-ray. Also, the assessment of each patients included history, skin prick test or CAP test, and sinus radiography. We compared the OSA-18 survey score and adenoidal-nasopharyngeal (AN) ratio between before and after treatment.
RESULTS
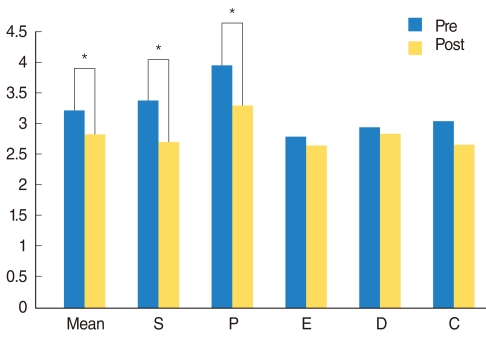
Total OSA-18 score and AN ratio decreased significantly after treatment regardless of allergy, sinusitis, and obesity (P=0.003, P=0.006). There was no complication after treatment of mometasone furoate.
CONCLUSION
Pediatric SDB patients with adenoid hypertrophy could be effectively treated with 4-weeks course of mometasone furoate. Allergy, obesity, and sinusitis did not affect on the result of treatment.
Keyword
MeSH Terms
Figure
Reference
-
1. Brouillette RT, Manoukian JJ, Ducharme FM, Oudjhane K, Earle LG, Ladan S, et al. Efficacy of fluticasone nasal spray for pediatric obstructive sleep apnea. J Pediatr. 2001; 6. 138(6):838–844. PMID: 11391326.
Article2. Alexopoulos EI, Kaditis AG, Kalampouka E, Kostadima E, Angelopoulos NV, Mikraki V, et al. Nasal corticosteroids for children with snoring. Pediatr Pulmonol. 2004; 8. 38(2):161–167. PMID: 15211701.
Article3. Gislason T, Benediktsdóttir B. Snoring, apneic episodes, and nocturnal hypoxemia among children 6 months to 6 years old: an epidemiologic study of lower limit of prevalence. Chest. 1995; 4. 107(4):963–966. PMID: 7705162.4. Sohn H, Rosenfeld RM. Evaluation of sleep-disordered breathing in children. Otolaryngol Head Neck Surg. 2003; 3. 128(3):344–352. PMID: 12646836.
Article5. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2) LEN and AllerGen). Allergy. 2008; 4. 63(Suppl 86):8–160. PMID: 18331513.6. Fokkens W, Lund V, Mullol J. European Position Paper on Rhinosinusitis and Nasal Polyps Group. EP3OS 2007: European position paper on rhinosinusitis and nasal polyps 2007. A summary for otorhinolaryngologists. Rhinology. 2007; 6. 45(2):97–101. PMID: 17708455.7. Silva VC, Leite AJ. Quality of life in children with sleep-disordered breathing: evaluation by OSA-18. Braz J Otorhinolaryngol. 2006; Nov–Dec. 72(6):747–756. PMID: 17308827.8. Strocker AM, Carrer A, Shapiro NL. The validity of the OSA-18 among three groups of pediatric patients. Int J Pediatr Otorhinolaryngol. 2005; 2. 69(2):241–247. PMID: 15656959.
Article9. Fujioka M, Young LW, Girdany BR. Radiographic evaluation of adenoidal size in children: adenoidal-nasopharyngeal ratio. AJR Am J Roentgenol. 1979; 9. 133(3):401–404. PMID: 111497.
Article10. Minshall E, Ghaffar O, Cameron L, O'Brien F, Quinn H, Rowe-Jones J, et al. Assessment by nasal biopsy of long-term use of mometasone furoate aqueous nasal spray (Nasonex) in the treatment of perennial rhinitis. Otolaryngol Head Neck Surg. 1998; 5. 118(5):648–654. PMID: 9591864.
Article11. Boner AL. Effects of intranasal corticosteroids on the hypothalamic-pituitary-adrenal axis in children. J Allergy Clin Immunol. 2001; 7. 108(1 Suppl):S32–S39. PMID: 11449204.
Article12. Schenkel EJ, Skoner DP, Bronsky EA, Miller SD, Pearlman DS, Rooklin A, et al. Absence of growth retardation in children with perennial allergic rhinitis after one year of treatment with mometasone furoate aqueous nasal spray. Pediatrics. 2000; 2. 105(2):E22. PMID: 10654982.
Article13. Szefler SJ. Pharmacokinetics of intranasal corticosteroids. J Allergy Clin Immunol. 2001; 7. 108(1 Suppl):S26–S31. PMID: 11449203.
Article14. Berlucchi M, Salsi D, Valetti L, Parrinello G, Nicolai P. The role of mometasone furoate aqueous nasal spray in the treatment of adenoidal hypertrophy in the pediatric age group: preliminary results of a prospective, randomized study. Pediatrics. 2007; 6. 119(6):e1392–e1397. PMID: 17533178.
Article15. Cohen LM, Koltai PJ, Scott JR. Lateral cervical radiographs and adenoid size: do they correlate? Ear Nose Throat J. 1992; 12. 71(12):638–642. PMID: 1483401.
Article16. Major MP, Flores-Mir C, Major PW. Assessment of lateral cephalometric diagnosis of adenoid hypertrophy and posterior upper airway obstruction: a systematic review. Am J Orthod Dentofacial Orthop. 2006; 12. 130(6):700–708. PMID: 17169731.
Article17. Cho JH, Lee DH, Lee NS, Won YS, Yoon HR, Suh BD. Size assessment of adenoid and nasopharyngeal airway by acoustic rhinometry in children. J Laryngol Otol. 1999; 10. 113(10):899–905. PMID: 10664704.
Article18. Piszcz M, Skotnicka B, Hassmann-Poznanska E. Acoustic rhinometry evaluation of adenoid hypertrophy and adenoidectomy efficacy. Otolaryngol Pol. 2008; 62(3):300–304. PMID: 18652154.19. Demain JG, Goetz DW. Pediatric adenoidal hypertrophy and nasal airway obstruction: reduction with aqueous nasal beclomethasone. Pediatrics. 1995; 3. 95(3):355–364. PMID: 7862473.
Article20. Cengel S, Akyol MU. The role of topical nasal steroids in the treatment of children with otitis media with effusion and/or adenoid hypertrophy. Int J Pediatr Otorhinolaryngol. 2006; 4. 70(4):639–645. PMID: 16169093.
Article21. Kimoff RJ, Hamid Q, Divangahi M, Hussain S, Bao W, Naor N, et al. Increased upper airway cytokines and oxidative stress in severe obstructive sleep apnoea. Eur Respir J. 2010; 9. 16. [Epub]. DOI: 10.1183/09031936.00048610.
Article22. Sabato R, Guido P, Salerno FG, Resta O, Spanevello A, Barbaro MP. Airway inflammation in patients affected by obstructive sleep apnea. Monaldi Arch Chest Dis. 2006; 6. 65(2):102–105. PMID: 16913581.
Article23. Franco RA Jr, Rosenfeld RM, Rao M. First place--resident clinical science award 1999: quality of life for children with obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000; 7. 123(1 Pt 1):9–16. PMID: 10889473.24. Mitchell RB, Kelly J. Quality of life after adenotonsillectomy for SDB in children. Otolaryngol Head Neck Surg. 2005; 10. 133(4):569–572. PMID: 16213931.
Article25. Mlynarek A, Tewfik MA, Hagr A, Manoukian JJ, Schloss MD, Tewfik TL, et al. Lateral neck radiography versus direct video rhinoscopy in assessing adenoid size. J Otolaryngol. 2004; 12. 33(6):360–365. PMID: 15971651.
Article26. Selkin SG. Flexible fiberoptics and pediatric otolaryngology: a simple technique for examination and photodocumentation. Int J Pediatr Otorhinolaryngol. 1983; 7. 5(3):325–333. PMID: 6629661.
Article27. Modrzyński M, Zawisza E, Mazurek H. The influence of medical treatment of the perennial allergic rhinitis on the adenoid size in children. Otolaryngol Pol. 2006; 60(4):543–550. PMID: 17152807.