Anat Cell Biol.
2013 Sep;46(3):177-182. 10.5115/acb.2013.46.3.177.
The expression of IL-6Ralpha and Gp130 in fallopian tubes bearing an ectopic pregnancy
- Affiliations
-
- 1Department of Biology and Anatomical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran. mghaffarin@yahoo.com
- 2Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
- KMID: 2263143
- DOI: http://doi.org/10.5115/acb.2013.46.3.177
Abstract
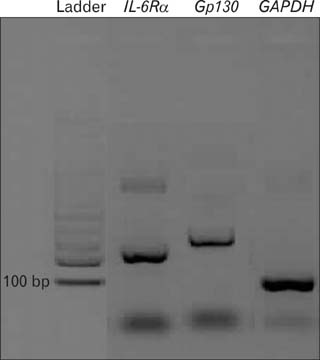
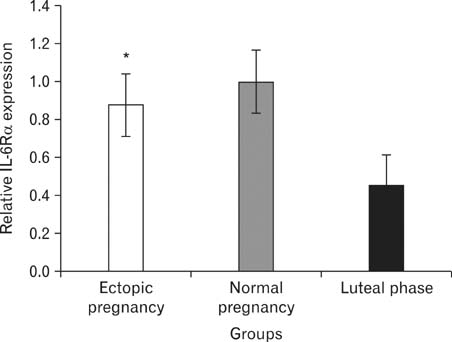
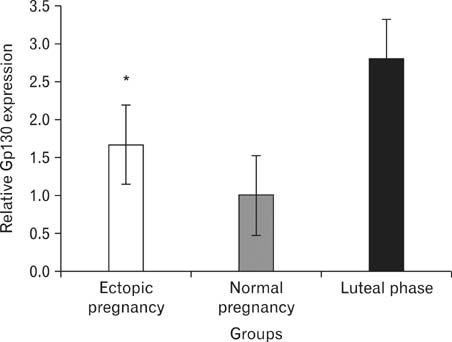
- Women with tubal ectopic pregnancies have high levels of circulating interleukin 6 (IL-6). IL-6 treatment in vitro significantly reduces the ciliary activity of tubal epithelium. The effects of IL-6 on target cells occur via the formation of a high-affinity complex with its receptors IL-6Ralpha and glycoprotein 130 (Gp130). IL-6Ralpha is specifically expressed in the cilia of the epithelial cells. In this study, we performed a quantitative reverse transcriptase polymerase chain reaction to determine the mRNA expression of IL-6Ralpha and Gp130 in the fallopian tubes obtained from 12 women with ectopic pregnancies, 12 women with normal pregnancies, and 12 healthy nonpregnant women in the luteal phase of their menstrual cycle. Fallopian tubes were evaluated from specimens taken during tubal ligation in normal pregnancies and nonpregnant fertile women or during tubal surgery in ectopic pregnancies. We observed that IL-6Ralpha mRNA expression in fallopian tubes was increased in ectopic pregnancy compared with that in the midluteal phase. We also found that the Gp130 mRNA expression was significantly lower in fallopian tubes from ectopic pregnancies than in those from nonpregnant women during the midluteal phase of their menstrual cycle, although its expression was noticeably high in fallopian tubes in the midluteal phase, which suggests that high Gp130 levels may possibly contribute to embryo transport into the uterus.
MeSH Terms
-
Cilia
Embryonic Structures
Epithelial Cells
Epithelium
Fallopian Tubes
Female
Glycoproteins
Humans
Interleukin-6
Luteal Phase
Menstrual Cycle
Pregnancy
Pregnancy, Ectopic
Receptors, Interleukin-6
Reverse Transcriptase Polymerase Chain Reaction
RNA, Messenger
Sterilization, Tubal
Ursidae
Uterus
Glycoproteins
Interleukin-6
RNA, Messenger
Receptors, Interleukin-6
Figure
Reference
-
1. Kriebs JM, Fahey JO. Ectopic pregnancy. J Midwifery Womens Health. 2006; 51:431–439.2. Bouyer J, Coste J, Fernandez H, Pouly JL, Job-Spira N. Sites of ectopic pregnancy: a 10 year population-based study of 1800 cases. Hum Reprod. 2002; 17:3224–3230.3. Kiran G, Kiran H, Ertopcu K, Kilinc M, Ekerbicer HC, Vardar MA. Tuba uterina leukemia inhibitory factor concentration does not increase in tubal pregnancy: a preliminary study. Fertil Steril. 2005; 83:484–486.4. Shao R, Zou S, Wang X, Feng Y, Brännström M, Stener-Victorin E, Billig H. Revealing the hidden mechanisms of smoke-induced fallopian tubal implantation. Biol Reprod. 2012; 86:131.5. Shao R. Understanding the mechanisms of human tubal ectopic pregnancies: new evidence from knockout mouse models. Hum Reprod. 2010; 25:584–587.6. Farquhar CM. Ectopic pregnancy. Lancet. 2005; 366:583–591.7. Shaw JL, Horne AW. The paracrinology of tubal ectopic pregnancy. Mol Cell Endocrinol. 2012; 358:216–222.8. Lyons RA, Saridogan E, Djahanbakhch O. The reproductive significance of human Fallopian tube cilia. Hum Reprod Update. 2006; 12:363–372.9. Orsi NM, Tribe RM. Cytokine networks and the regulation of uterine function in pregnancy and parturition. J Neuroendocrinol. 2008; 20:462–469.10. Dimitriadis E, White CA, Jones RL, Salamonsen LA. Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod Update. 2005; 11:613–630.11. Pfeilschifter J, Köditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002; 23:90–119.12. Machelon V, Emilie D, Lefevre A, Nome F, Durand-Gasselin I, Testart J. Interleukin-6 biosynthesis in human preovulatory follicles: some of its potential roles at ovulation. J Clin Endocrinol Metab. 1994; 79:633–642.13. Papathanasiou A, Djahanbakhch O, Saridogan E, Lyons RA. The effect of interleukin-6 on ciliary beat frequency in the human fallopian tube. Fertil Steril. 2008; 90:391–394.14. Balasubramaniam ES, Van Noorden S, El-Bahrawy M. The expression of interleukin (IL)-6, IL-8, and their receptors in fallopian tubes with ectopic tubal gestation. Fertil Steril. 2012; 98:898–904.15. Shao R, Nutu M, Karlsson-Lindahl L, Benrick A, Weijdegård B, Lager S, Egecioglu E, Fernandez-Rodriguez J, Gemzell-Danielsson K, Ohlsson C, Jansson JO, Billig H. Downregulation of cilia-localized Il-6R alpha by 17beta-estradiol in mouse and human fallopian tubes. Am J Physiol Cell Physiol. 2009; 297:C140–C151.16. Lee BS, Choi JY, Cha JH. Expression of ciliary neurotrophic factor and its receptor in experimental obstructive nephropathy. Anat Cell Biol. 2011; 44:85–97.17. Al-Azemi M, Refaat B, Amer S, Ola B, Chapman N, Ledger W. The expression of inducible nitric oxide synthase in the human fallopian tube during the menstrual cycle and in ectopic pregnancy. Fertil Steril. 2010; 94:833–840.18. Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975; 122:262–263.19. Refaat B, Amer S, Ola B, Chapman N, Ledger W. The expression of activin-betaA- and -betaB-subunits, follistatin, and activin type II receptors in fallopian tubes bearing an ectopic pregnancy. J Clin Endocrinol Metab. 2008; 93:293–299.20. Lam PM, Briton-Jones C, Cheung CK, Leung SW, Cheung LP, Haines C. Increased messenger RNA expression of vascular endothelial growth factor and its receptors in the implantation site of the human oviduct with ectopic gestation. Fertil Steril. 2004; 82:686–690.21. Zachariades E, Foster H, Goumenou A, Thomas P, Rand-Weaver M, Karteris E. Expression of membrane and nuclear progesterone receptors in two human placental choriocarcinoma cell lines (JEG-3 and BeWo): effects of syncytialization. Int J Mol Med. 2011; 27:767–774.22. Halbert SA, Tam PY, Blandau RJ. Egg transport in the rabbit oviduct: the roles of cilia and muscle. Science. 1976; 191:1052–1053.23. Halbert SA, Becker DR, Szal SE. Ovum transport in the rat oviductal ampulla in the absence of muscle contractility. Biol Reprod. 1989; 40:1131–1136.24. Kishimoto T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther. 2006; 8:Suppl 2. S2.25. Leese HJ, Tay JI, Reischl J, Downing SJ. Formation of Fallopian tubal fluid: role of a neglected epithelium. Reproduction. 2001; 121:339–346.26. Vasquez G, Winston RM, Brosens IA. Tubal mucosa and ectopic pregnancy. Br J Obstet Gynaecol. 1983; 90:468–474.27. Evans J, Catalano RD, Brown P, Sherwin R, Critchley HO, Fazleabas AT, Jabbour HN. Prokineticin 1 mediates fetal-maternal dialogue regulating endometrial leukemia inhibitory factor. FASEB J. 2009; 23:2165–2175.28. Jabbour HN, Sales KJ, Catalano RD, Norman JE. Inflammatory pathways in female reproductive health and disease. Reproduction. 2009; 138:903–919.29. Li HZ, Sun X, Stavreus-Evers A, Gemzell-Danielsson K. Effect of mifepristone on the expression of cytokines in the human Fallopian tube. Mol Hum Reprod. 2004; 10:489–493.30. Senturk LM, Arici A. Leukemia inhibitory factor in human reproduction. Am J Reprod Immunol. 1998; 39:144–151.31. Shao R, Egecioglu E, Weijdegård B, Kopchick JJ, Fernandez-Rodriguez J, Andersson N, Billig H. Dynamic regulation of estrogen receptor-alpha isoform expression in the mouse fallopian tube: mechanistic insight into estrogen-dependent production and secretion of insulin-like growth factors. Am J Physiol Endocrinol Metab. 2007; 293:E1430–E1442.32. Shao R, Ljungström K, Weijdegård B, Egecioglu E, Fernandez-Rodriguez J, Zhang FP, Thurin-Kjellberg A, Bergh C, Billig H. Estrogen-induced upregulation of AR expression and enhancement of AR nuclear translocation in mouse fallopian tubes in vivo. Am J Physiol Endocrinol Metab. 2007; 292:E604–E614.33. Shao R, Feng Y, Zou S, Weijdegård B, Wu G, Brännström M, Billig H. The role of estrogen in the pathophysiology of tubal ectopic pregnancy. Am J Transl Res. 2012; 4:269–278.34. Srivastava MD, Lippes J, Srivastava BI. Cytokines of the human reproductive tract. Am J Reprod Immunol. 1996; 36:157–166.35. Wanggren K, Lalitkumar PG, Hambiliki F, Ståbi B, Gemzell-Danielsson K, Stavreus-Evers A. Leukaemia inhibitory factor receptor and gp130 in the human Fallopian tube and endometrium before and after mifepristone treatment and in the human preimplantation embryo. Mol Hum Reprod. 2007; 13:391–397.36. Austgulen R, Arntzen KJ, Vatten LJ, Kahn J, Sunde A. Detection of cytokines (interleukin-1, interleukin-6, transforming growth factor-beta) and soluble tumour necrosis factor receptors in embryo culture fluids during in-vitro fertilization. Hum Reprod. 1995; 10:171–176.