Allergy Asthma Respir Dis.
2014 May;2(2):138-141. 10.4168/aard.2014.2.2.138.
Delayed onset urticaria and angioedema caused by components of itraconazole solution
- Affiliations
-
- 1Department of Internal Medicine, Kosin University College of Medicine, Busan, Korea. soonichoi@gmail.com
- KMID: 2261750
- DOI: http://doi.org/10.4168/aard.2014.2.2.138
Abstract
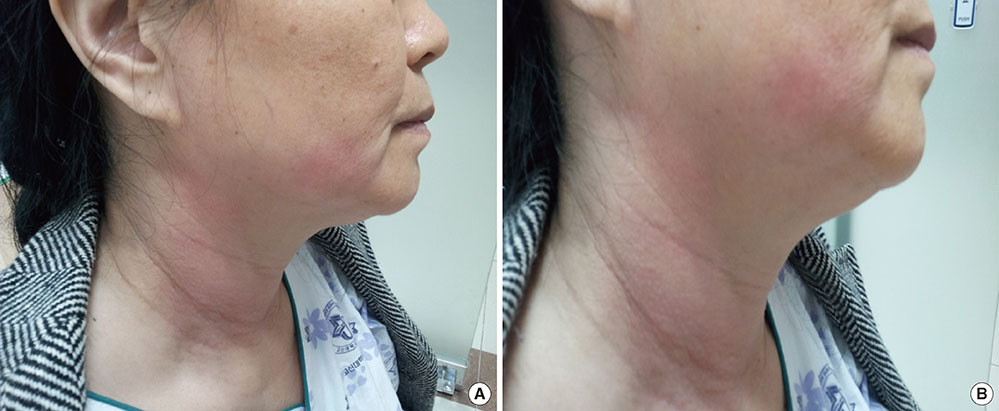
- Itraconazole, new triazole agent with a broader antifungal spectrum than fluconazole, has been prescribed widely in the treatment and prophylaxis for fungal infection. Itaconazole has been reported to have gastrointestinal disturbance (4%) and headache (1%) as its most common side-effects. However, allergic reactions caused by this drug are rare. A 53-year-old woman with myelodysplastic syndrome received prophylactic antibiotic therapy including itraconazole solution before chemotherapy. She complained of hive on the face with angioedema at 6 hours after taking them. The symptoms were more aggravated on the next day and reversed by stopping itraconazole solution and injection of antihistamine and steroids. Skin prick tests with itraconazole solution, itraconazole tablet, and ketoconazole showed all the negative responses. The oral challenge test with itraconazole solution was performed and resulted in urticaria and angioedema 6 hours later. Next, the oral challenge test with intraconazole tablet was performed and showed negative response. The patient was finally diagnosed as adverse reaction by additives contained intraconazole solution. We report, a case of delayed onset urticaria and angioedema caused by components of itraconazole solution.
Keyword
MeSH Terms
Figure
Reference
-
1. De Beule K, Van Gestel J. Pharmacology of itraconazole. Drugs. 2001; 61:Suppl 1. 27–37.
Article2. Bodey GP. Azole antifungal agents. Clin Infect Dis. 1992; 14:Suppl 1. S161–S169.
Article3. Tucker RM, Haq Y, Denning DW, Stevens DA. Adverse events associated with itraconazole in 189 patients on chronic therapy. J Antimicrob Chemother. 1990; 26:561–566.
Article4. Degreef H. New antifungal agents in the treatment of superficial dermatomycoses. Ann Dermatol Venereol. 1993; 120:21–31.5. Saag MS, Dismukes WE. Azole antifungal agents: emphasis on new triazoles. Antimicrob Agents Chemother. 1988; 32:1–8.
Article6. Hostetler JS, Hanson LH, Stevens DA. Effect of cyclodextrin on the pharmacology of antifungal oral azoles. Antimicrob Agents Chemother. 1992; 36:477–480.
Article7. Chang CH, Young-Xu Y, Kurth T, Orav JE, Chan AK. The safety of oral antifungal treatments for superficial dermatophytosis and onychomycosis: a meta-analysis. Am J Med. 2007; 120:791–798.
Article8. Chen SC, Sorrell TC. Antifungal agents. Med J Aust. 2007; 187:404–409.
Article9. Amichai B, Grunwald MH. Adverse drug reactions of the new oral antifungal agents: terbinafine, fluconazole, and itraconazole. Int J Dermatol. 1998; 37:410–415.
Article10. Gupta A, Lambert J, Revuz J, Shear N. Update on the safety of itraconazole pulse therapy in onychomycosis and dermatomycoses. Eur J Dermatol. 2001; 11:6–10.11. Batinac T, Zamolo G, Troselj-Vukie B, Biljan D, Petranovic D, Kujundzic M. Maculopapular eruption secondary to itraconazole. Coll Antropol. 2008; 32:649–651.12. Kang MC, Kim SA, Lee KS, Cho JW. Acute generalized exanthematous pustulosis induced by itraconazole. Korean J Dermatol. 2009; 47:1098–1101.13. Kim SI, Park TH, Yoo JH, Kim KJ. A case of drug eruption caused by itraconazole. Korean J Dermatol. 1999; 37:1700–1702.14. Gonzalo MA, de Argila D, García JM, Alvarado MI. Allergic contact dermatitis to propylene glycol. Allergy. 1999; 54:82–83.
Article15. Sowa J, Suzuki K, Tsuruta K, Akamatsu H, Matsunaga K. Allergic contact dermatitis from propylene glycol ricinoleate in a lipstick. Contact Dermatitis. 2003; 48:228–229.
Article16. Baldo BA, McDonnell NJ, Pham NH. Drug-specific cyclodextrins with emphasis on sugammadex, the neuromuscular blocker rocuronium and perioperative anaphylaxis: implications for drug allergy. Clin Exp Allergy. 2011; 41:1663–1678.
Article17. Asarch A, Scheinman PL. Sorbitan sesquioleate: an emerging contact allergen. Dermatitis. 2008; 19:339–341.
Article18. Schmutz JL, Barbaud A, Trechot P. Urticaria and angiodema due to itraconazole. Ann Dermatol Venereol. 2005; 132:403.19. Martínez-Alonso JC, Domínguez-Ortega FJ, Fuentes-Gonzalo MJ. Urticaria and angioedema due to itraconazole. Allergy. 2003; 58:1317–1318.
Article20. Reshef A, Kagey-Sobotka A, Adkinson NF Jr, Lichtenstein LM, Norman PS. The pattern and kinetics in human skin of erythema and mediators during the acute and late-phase response (LPR). J Allergy Clin Immunol. 1989; 84(5 Pt 1):678–687.
Article