Allergy Asthma Immunol Res.
2013 Mar;5(2):96-101. 10.4168/aair.2013.5.2.96.
The Influence of the Time and Temperature of Heat Treatment on the Allergenicity of Egg White Proteins
- Affiliations
-
- 1Department of Pediatrics, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea.
- 2Environmental Health Center for Atopic Dermatitis, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. kmaped@skku.edu
- KMID: 2260344
- DOI: http://doi.org/10.4168/aair.2013.5.2.96
Abstract
- PURPOSE
The present study was performed to determine the factor, either duration or the temperature of heat treatment, exerting maximal and significant influence on the composition and allergenicity of egg white (EW) proteins.
METHODS
Raw EW and 4 kinds of heated EW (fried EW, boiled EW for 10 minutes, boiled EW for 30 minutes, and baked EW for 20 minutes at 170degrees C) were prepared, and subsequently protein extraction was carried out. The proteins were separated by SDS-PAGE, and then immunoglobulin E (IgE) immunoblots were performed with the sera of 7 egg-allergic patients. Furthermore, the antigenic activities of ovalbumin (OVA), ovomucoid (OM), and ovotransferrin (OT) in different EW samples were measured by inhibition enzyme-linked Immuno-sorbent assay (ELISA).
RESULTS
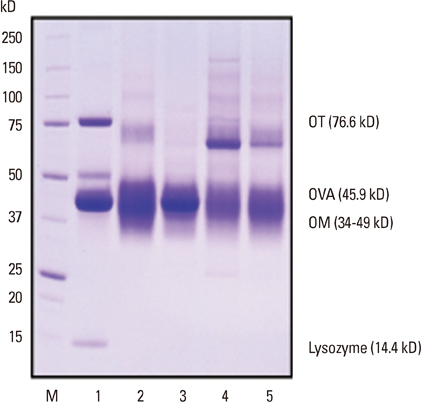
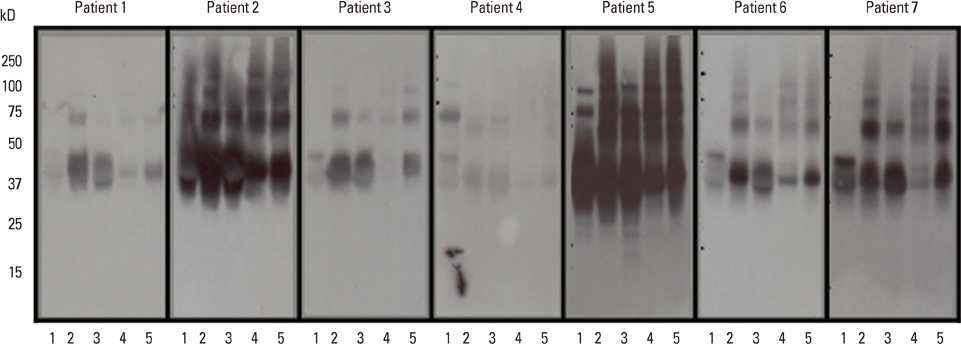
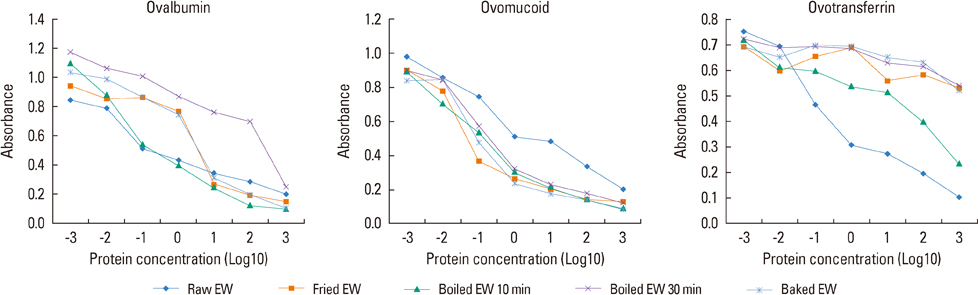
In SDS-PAGE analysis, the intensity of the protein band at 45 kD (corresponding to OVA) decreased significantly in boiled EW (30 minutes) and baked EW, but no change was observed in the case of boiled EW for 10 minutes. In IgE immunoblots, the IgE response to 34-50 kD (OM and OVA) in boiled EW for 30 minutes decreased significantly, when compared with raw EW and other heated EWs. In inhibition ELISA, a significant decrease in the OVA antigenic activity was observed in boiled EW for 30 minutes amongst other heated EW samples. However, OM antigenic activity in all kinds of heated EW including boiled EW for 30 minutes did not reduce after heat treatment. The OT antigenic activity nearly disappeared in heated EWs except in the case of boiled EW for 10 minutes.
CONCLUSIONS
Amongst 4 kinds of heated EWs, the boiled EW for 30 minutes showed the most significant changes both in composition and reduction in allergenicity. Our results revealed that the duration of heat treatment had more influence on the composition and allergenicity of EW proteins than the temperature.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Update on egg allergy in children
Meeyong Shin
Allergy Asthma Respir Dis. 2015;3(1):15-21. doi: 10.4168/aard.2015.3.1.15.
Reference
-
1. Bock SA, Atkins FM. Patterns of food hypersensitivity during sixteen years of double-blind, placebo-controlled food challenges. J Pediatr. 1990. 117:561–567.2. Boyano-Martínez T, García-Ara C, Díaz-Pena JM, Martín-Esteban M. Prediction of tolerance on the basis of quantification of egg white-specific IgE antibodies in children with egg allergy. J Allergy Clin Immunol. 2002. 110:304–309.3. Sicherer SH, Sampson HA. 9. Food allergy. J Allergy Clin Immunol. 2006. 117:S470–S475.4. Eggesbø M, Botten G, Halvorsen R, Magnus P. The prevalence of allergy to egg: a population-based study in young children. Allergy. 2001. 56:403–411.5. Sampson HA, McCaskill CC. Food hypersensitivity and atopic dermatitis: evaluation of 113 patients. J Pediatr. 1985. 107:669–675.6. Niggemann B, Sielaff B, Beyer K, Binder C, Wahn U. Outcome of double-blind, placebo-controlled food challenge tests in 107 children with atopic dermatitis. Clin Exp Allergy. 1999. 29:91–96.7. Anet J, Back JF, Baker RS, Barnett D, Burley RW, Howden ME. Allergens in the white and yolk of hen's egg. A study of IgE binding by egg proteins. Int Arch Allergy Appl Immunol. 1985. 77:364–371.8. Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol. 1997. 100:444–451.9. Langeland T. A clinical and immunological study of allergy to hen's egg white. III. Allergens in hen's egg white studied by crossed radio-immunoelectrophoresis (CRIE). Allergy. 1982. 37:521–530.10. Hoffman DR. Immunochemical identification of the allergens in egg white. J Allergy Clin Immunol. 1983. 71:481–486.11. Holen E, Elsayed S. Characterization of four major allergens of hen egg-white by IEF/SDS-PAGE combined with electrophoretic transfer and IgE-immunoautoradiography. Int Arch Allergy Appl Immunol. 1990. 91:136–141.12. Bernhisel-Broadbent J, Dintzis HM, Dintzis RZ, Sampson HA. Allergenicity and antigenicity of chicken egg ovomucoid (Gal d III) compared with ovalbumin (Gal d I) in children with egg allergy and in mice. J Allergy Clin Immunol. 1994. 93:1047–1059.13. Urisu A, Ando H, Morita Y, Wada E, Yasaki T, Yamada K, Komada K, Torii S, Goto M, Wakamatsu T. Allergenic activity of heated and ovomucoid-depleted egg white. J Allergy Clin Immunol. 1997. 100:171–176.14. Cooke SK, Sampson HA. Allergenic properties of ovomucoid in man. J Immunol. 1997. 159:2026–2032.15. Joo K, Kato Y. Assessment of allergenic activity of a heat-coagulated ovalbumin after in vivo digestion. Biosci Biotechnol Biochem. 2006. 70:591–597.16. Coombs RR, McLaughlan P. Allergenicity of food proteins and its possible modification. Ann Allergy. 1984. 53:592–596.17. Davis PJ, Williams SC. Protein modification by thermal processing. Allergy. 1998. 53:102–105.18. Desert C, Guérin-Dubiard C, Nau F, Jan G, Val F, Mallard J. Comparison of different electrophoretic separations of hen egg white proteins. J Agric Food Chem. 2001. 49:4553–4561.19. Peng HJ, Chang ZN, Tsai LC, Su SN, Shen HD, Chang CH. Heat denaturation of egg-white proteins abrogates the induction of oral tolerance of specific Th2 immune responses in mice. Scand J Immunol. 1998. 48:491–496.20. Mine Y, Zhang JW. Comparative studies on antigenicity and allergenicity of native and denatured egg white proteins. J Agric Food Chem. 2002. 50:2679–2683.21. Nowak-Wegrzyn A, Bloom KA, Sicherer SH, Shreffler WG, Noone S, Wanich N, Sampson HA. Tolerance to extensively heated milk in children with cow's milk allergy. J Allergy Clin Immunol. 2008. 122:342.e1–347.e2.22. Lemon-Mulé H, Sampson HA, Sicherer SH, Shreffler WG, Noone S, Nowak-Wegrzyn A. Immunologic changes in children with egg allergy ingesting extensively heated egg. J Allergy Clin Immunol. 2008. 122:977–983.e1.23. Kemp AS. Egg allergy. Pediatr Allergy Immunol. 2007. 18:696–702.24. Eigenmann PA. Anaphylactic reactions to raw eggs after negative challenges with cooked eggs. J Allergy Clin Immunol. 2000. 105:587–588.25. Burks AW Jr, Brooks JR, Sampson HA. Allergenicity of major component proteins of soybean determined by enzyme-linked immunosorbent assay (ELISA) and immunoblotting in children with atopic dermatitis and positive soy challenges. J Allergy Clin Immunol. 1988. 81:1135–1142.26. Des Roches A, Nguyen M, Paradis L, Primeau MN, Singer S. Tolerance to cooked egg in an egg allergic population. Allergy. 2006. 61:900–901.27. Wood RA. The natural history of food allergy. Pediatrics. 2003. 111:1631–1637.28. Savage JH, Matsui EC, Skripak JM, Wood RA. The natural history of egg allergy. J Allergy Clin Immunol. 2007. 120:1413–1417.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Influence of the Presence of Wheat Flour on the Antigenic Activities of Egg White Proteins
- Hyperresponsiveness to Boiled Egg Yolk in Early Life Leads to Prolonged Egg Allergy
- Variation in the Allergenicity of Scrambled, Boiled, Short-Baked and Long-Baked Egg White Proteins
- The Role of Heat Shock Protein in Perinatal Fields
- Heat Shock Protein Induction By An Infrared Warm Compression Device