Allergy Asthma Immunol Res.
2013 Jul;5(4):224-231. 10.4168/aair.2013.5.4.224.
Effect of Prostaglandin E2 on Vascular Endothelial Growth Factor Production in Nasal Polyp Fibroblasts
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Soonchunhyang University College of Medicine, Cheonan Hospital, Cheonan, Korea. bjbaek@schmc.ac.kr
- 2Division of Brain Korea 21 Program for Biomedical Science, Korea University College of Medicine, Seoul, Korea.
- 3Department of Otorhinolaryngology-Head and Neck Surgery, Korea University College of Medicine, Seoul, Korea.
- KMID: 2260316
- DOI: http://doi.org/10.4168/aair.2013.5.4.224
Abstract
- PURPOSE
Angiogenesis is involved in the pathogenesis of chronic rhinosinusitis with nasal polyps. We aimed to investigate the effects of prostaglandin E2 (PGE2) on vascular endothelial growth factor (VEGF) production, the role of E-prostanoid (EP) 4 receptors, and the signal transduction pathway mediating VEGF production in nasal polyp-derived fibroblasts (NPDFs).
METHODS
Eight primary NPDF cultures were established from nasal polyps, which were incubated with or without PGE2. Reverse transcription-polymerase chain reaction amplification of EP receptors (EP1, EP2, EP3, and EP4) and immunofluorescence staining for VEGF production were performed. VEGF production via the cyclic adenosine monophosphate (cAMP)-dependent protein kinase A (PKA) and phosphatidylinositol 3-kinase (PI3K) pathways was evaluated by enzyme-linked immunosorbent assay.
RESULTS
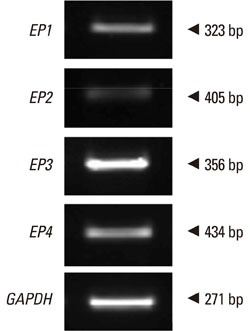
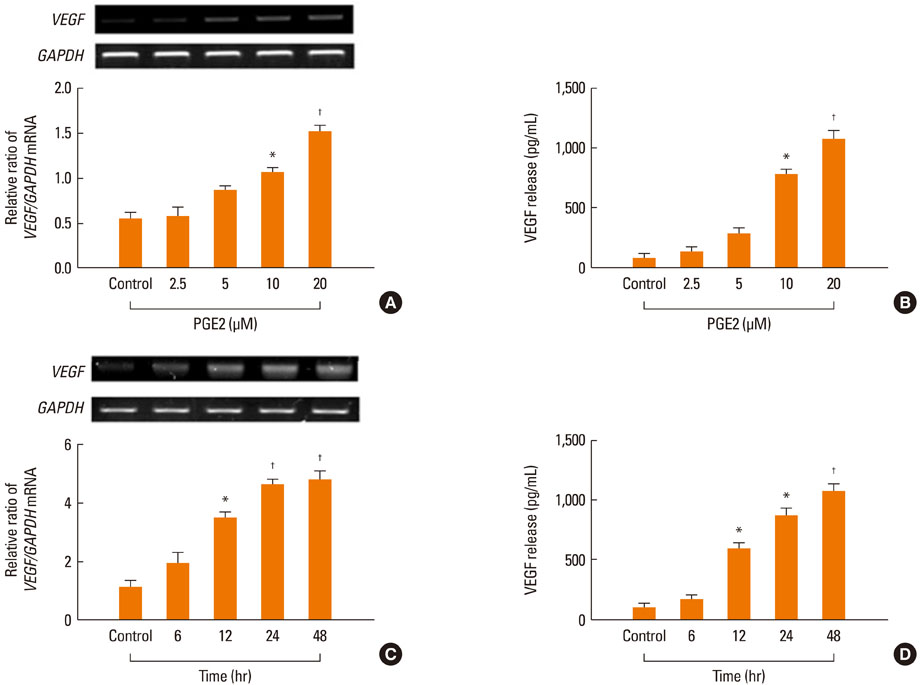
All EP receptors were expressed in NPDFs. PGE2 significantly increased VEGF production concentration- and time dependently, and VEGF production was regulated by an EP4 receptor. Activation of intracellular cAMP regulated VEGF production. VEGF production was decreased by PKA and PI3K inhibitors via intracellular cAMP.
CONCLUSIONS
PGE2 stimulates VEGF production via the EP4 receptor in NPDFs. These results indicate that PGE2-induced VEGF production is mediated, at least partially, through cAMP-dependent signaling pathways. Therapies targeting the EP4 receptor may be effective in inhibiting the development of nasal polyps.
Keyword
MeSH Terms
-
Adenosine Monophosphate
Cyclic AMP-Dependent Protein Kinases
Dinoprostone
Fibroblasts
Fluorescent Antibody Technique
Nasal Polyps
Negotiating
Phosphatidylinositol 3-Kinase
Signal Transduction
Vascular Endothelial Growth Factor A
Adenosine Monophosphate
Cyclic AMP-Dependent Protein Kinases
Dinoprostone
Phosphatidylinositol 3-Kinase
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Coste A, Brugel L, Maître B, Boussat S, Papon JF, Wingerstmann L, Peynègre R, Escudier E. Inflammatory cells as well as epithelial cells in nasal polyps express vascular endothelial growth factor. Eur Respir J. 2000; 15:367–372.2. Biteman B, Hassan IR, Walker E, Leedom AJ, Dunn M, Seta F, Laniado-Schwartzman M, Gronert K. Interdependence of lipoxin A4 and heme-oxygenase in counter-regulating inflammation during corneal wound healing. FASEB J. 2007; 21:2257–2266.3. Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest. 2001; 108:15–23.4. Cho KN, Choi JY, Kim CH, Baek SJ, Chung KC, Moon UY, Kim KS, Lee WJ, Koo JS, Yoon JH. Prostaglandin E2 induces MUC8 gene expression via a mechanism involving ERK MAPK/RSK1/cAMP response element binding protein activation in human airway epithelial cells. J Biol Chem. 2005; 280:6676–6681.5. Yoshimura T, Yoshikawa M, Otori N, Haruna S, Moriyama H. Correlation between the prostaglandin D(2)/E(2) ratio in nasal polyps and the recalcitrant pathophysiology of chronic rhinosinusitis associated with bronchial asthma. Allergol Int. 2008; 57:429–436.6. Peacock CD, Misso NL, Watkins DN, Thompson PJ. PGE 2 and dibutyryl cyclic adenosine monophosphate prolong eosinophil survival in vitro. J Allergy Clin Immunol. 1999; 104:153–162.7. Ben-Av P, Crofford LJ, Wilder RL, Hla T. Induction of vascular endothelial growth factor expression in synovial fibroblasts by prostaglandin E and interleukin-1: a potential mechanism for inflammatory angiogenesis. FEBS Lett. 1995; 372:83–87.8. Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007; 282:11613–11617.9. Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001; 41:661–690.10. Hatazawa R, Tanigami M, Izumi N, Kamei K, Tanaka A, Takeuchi K. Prostaglandin E2 stimulates VEGF expression in primary rat gastric fibroblasts through EP4 receptors. Inflammopharmacology. 2007; 15:214–217.11. Inoue H, Takamori M, Shimoyama Y, Ishibashi H, Yamamoto S, Koshihara Y. Regulation by PGE2 of the production of interleukin-6, macrophage colony stimulating factor, and vascular endothelial growth factor in human synovial fibroblasts. Br J Pharmacol. 2002; 136:287–295.12. Cheng T, Cao W, Wen R, Steinberg RH, LaVail MM. Prostaglandin E2 induces vascular endothelial growth factor and basic fibroblast growth factor mRNA expression in cultured rat Muller cells. Invest Ophthalmol Vis Sci. 1998; 39:581–591.13. Bradbury D, Clarke D, Seedhouse C, Corbett L, Stocks J, Knox A. Vascular endothelial growth factor induction by prostaglandin E2 in human airway smooth muscle cells is mediated by E prostanoid EP2/EP4 receptors and SP-1 transcription factor binding sites. J Biol Chem. 2005; 280:29993–30000.14. Sales KJ, Maudsley S, Jabbour HN. Elevated prostaglandin EP2 receptor in endometrial adenocarcinoma cells promotes vascular endothelial growth factor expression via cyclic 3',5'-adenosine monophosphate-mediated transactivation of the epidermal growth factor receptor and extracellular signal-regulated kinase 1/2 signaling pathways. Mol Endocrinol. 2004; 18:1533–1545.15. Fujino H, Salvi S, Regan JW. Differential regulation of phosphorylation of the cAMP response element-binding protein after activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. Mol Pharmacol. 2005; 68:251–259.16. Bateman ND, Fahy C, Woolford TJ. Nasal polyps: still more questions than answers. J Laryngol Otol. 2003; 117:1–9.17. Pawankar R. Nasal polyposis: an update: editorial review. Curr Opin Allergy Clin Immunol. 2003; 3:1–6.18. Wang QP, Escudier E, Roudot-Thoraval F, Abd-Al Samad I, Peynegre R, Coste A. Myofibroblast accumulation induced by transforming growth factor-beta is involved in the pathogenesis of nasal polyps. Laryngoscope. 1997; 107:926–931.19. Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002; 23:144–150.20. Samuelsson B, Morgenstern R, Jakobsson PJ. Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol Rev. 2007; 59:207–224.21. Sheng H, Shao J, Kirkland SC, Isakson P, Coffey RJ, Morrow J, Beauchamp RD, DuBois RN. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest. 1997; 99:2254–2259.22. Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999; 79:1193–1226.23. Kohyama T, Ertl RF, Valenti V, Spurzem J, Kawamoto M, Nakamura Y, Veys T, Allegra L, Romberger D, Rennard SI. Prostaglandin E(2) inhibits fibroblast chemotaxis. Am J Physiol Lung Cell Mol Physiol. 2001; 281:L1257–L1263.24. Arosh JA, Banu SK, Chapdelaine P, Emond V, Kim JJ, MacLaren LA, Fortier MA. Molecular cloning and characterization of bovine prostaglandin E2 receptors EP2 and EP4: expression and regulation in endometrium and myometrium during the estrous cycle and early pregnancy. Endocrinology. 2003; 144:3076–3091.25. Fujino H, West KA, Regan JW. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J Biol Chem. 2002; 277:2614–2619.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Basic Fibroblast Growth Factor(bFGF), Vascular Endothelial Growth Factor(VEGF), and Platelet-Derived Endothelial Cell Growth Factor(PD-ECGF) in Nasal Polyps
- Expression of Vascular Endothelial Growth Factor and Fibronectin in Nasal Polyps: Effects of Topical Corticosteroid
- The Effect of Cigarette Smoking on the Expression of MMP-9, TIMP-1 and VEGF in Nasal Polyp Epithelial Cells and Fibroblasts
- Distribution of Histologic Type of Nasal Polyp and Expression of Vascular Endothelial Growth Factor According to Nasal Polyp Type
- Expression of Vascular Endothelial Growth Factor and Fibronectin in Nasal Polyps: Effects of Topical Corticosteroid