Korean J Hematol.
2009 Mar;44(1):62-66. 10.5045/kjh.2009.44.1.62.
Complete Hematologic Response and Cytogenetic Remission after Imatinib and Dexamethasone Treatment of a Ph+ Precursor B-cell Acute Lymphoblastic Leukemia in Renal Transplantation Patient
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea.
- 2Department of Internal Medicine, Kyung Hee University Medical Center, Seoul, Korea. ksamcho@khmc.or.kr
- KMID: 2252177
- DOI: http://doi.org/10.5045/kjh.2009.44.1.62
Abstract
- In this report, we present a case of a patient with Philadelphia chromosome-positive (Ph+) B-cell acute lymphoblastic leukemia after renal transplantation. The patient, a 65-year-old man, had received a kidney transplantation 20 years prior to diagnosis with Ph+ precursor B-cell ALL. Because he was refractory to intensive chemotherapy and had refused to receive additional intensive chemotherapy, he was treated with imatinib and dexamethasone. While this patient experienced a complete hematologic and cytogenetic response, he did not show a complete molecular remission. Eighty days after imatinib combination therapy, the patient relapsed and died from intracerebral hemorrhage.
MeSH Terms
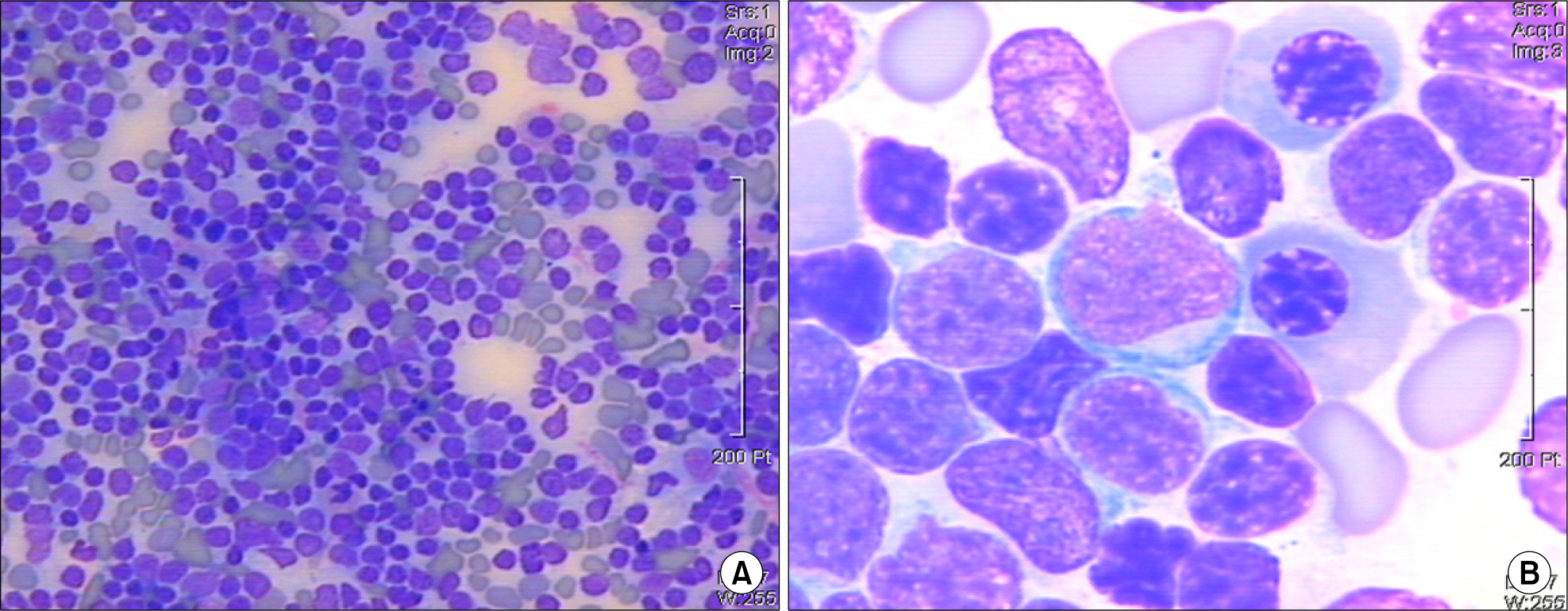
Figure
Reference
-
References
1. Penn I. Posttransplant malignancies. Transplant Proc. 1999; 31:1260–2.
Article2. Saadat A, Einollahi B, Ahmadzad-Asl MA, et al. Posttransplatation lymphoproliferative disorders in renal transplant recipients: report of over 20 years of experience. Transplantation Proc. 2007; 39:1071–3.3. Caillard S, Agodoa LY, Bohen EM, Abbott KC. Myeloma, Hodgkin disease, and lymphoid leukemia after renal transplantation: characteristics, risk factors and prognosis. Transplantation. 2006; 81:888–95.
Article4. Kantarjian HM, O'Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000; 18:547–61.
Article5. Sheil AG. ANZDATA (Australia and New Zealand Dialysis and Transplant Reistry) The 24th annal report 9: cancer report, 2002 (Accessed at. http://www.anzdata.org.au/anzdata/AnzdataReport/24thReport.6. Hojo M, Morimoto T, Maluccio M, et al. Cyclosporin induces cancer progression by a cell-autonomous mechanism. Nature. 1999; 397:530–4.7. McGeown MG, Douglas JF, Middleton D. One thousand renal transplants at Belfast City Hospital: post-graft neoplasia 1968–1999, comparing azathioprine only with cyclosporine-based regimens in a single centre. Clin Transpl. 2000; 16:193–202.8. Savage DG, Antman KH. Imatinib mesylate – a new oral targeted therapy. N Engl J Med. 2002; 346:683–93.
Article9. Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001; 344:1038–42.
Article10. Ottmann OG, Druker BJ, Sawyers CL, et al. A phase 2 study of imatinib in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoid leukemia. Blood. 2002; 100:1965–71.11. Wassmann B, Pfeifer H, Scheuring U, et al. Therapy with imatinib mesylate (Glivec) preceding allogeneic stem cell transplantation (SCT) in relapsed or refractory Philadelphia positive acute lymphoblastic leukemia (Ph+ ALL). Leukemia. 2002; 16:2358–65.12. Annino L, Vegna ML, Camera A, et al. Treatment of adult acute lymphoblastic leukemia (ALL): longterm fellow-up of the GIMEMA ALL 0288 randomized study. Blood. 2002; 99:863–71.13. Vignetti M, Fazi P, Cimino G, et al. Imatinib plus steroids induces complete remissions and prolonged survival in elderly Philadelphia chromosome-positive patients with acute lymphoblastic leukemia without additional chemotherapy: results of the Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) LAL0201-B protocol. Blood. 2007; 109:3676–8.
Article14. Wassmann B, Gökbuget N, Scheuring UJ, et al. A randomized multicenter open label phase II study to determine the safety and efficacy of induction therapy with imatinib (Glivec, formerly STI571) in comparison with standard induction chemotherapy in elderly (>55 years) patients with Philadelphia chromosome-positive (Ph+/BCR-ABL+) acute lymphoblastic leukemia (ALL) (CSTI571ADE 10). Ann Hematol. 2003; 82:716–20.15. Ottmann O, Dombret H, Martinelli G, et al. Dasatinib induces rapid hematologic and cytogenetic responses in adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia with resistance or intolerance to imatinib: interim results of a phase 2 study. Blood. 2007; 110:2309–15.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Complete Remission by Imatinib Mesylate (Glivec) in a Child Relapsing with Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia (Ph (+) ALL) after Unrelated Donor Stem Cell Transplantation
- Complete remission of philadelphia chromosome-positive acute myeloid leukemia with imatinib mesylate
- Precursor B-Cell Acute Lymphoblastic Leukemia in Two Patients with a History of Cytotoxic Therapy
- Philadelphia chromosome-positive acute lymphoblastic leukemia in childhood
- Acute Precursor T Cell Lymphoblastic Leukemia Associated with Behcet's Disease: A Case Report