Korean J Hematol.
2010 Mar;45(1):14-22. 10.5045/kjh.2010.45.1.14.
Quality assessment of cellular therapies: the emerging role of molecular assays
- Affiliations
-
- 1Cellular Therapy and Immunogenetics Sections, Department of Transfusion Medicine, Clinical Center, NIH, Bethesda, MD, USA. dstroncek@cc.nih.gov
- KMID: 2252078
- DOI: http://doi.org/10.5045/kjh.2010.45.1.14
Abstract
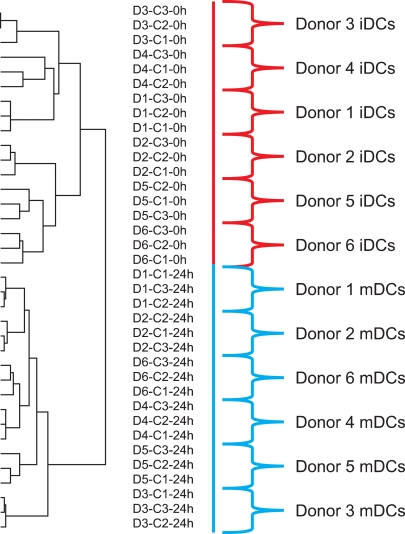
- Cellular therapies are becoming increasingly important in treating cancer, hematologic malignancies, autoimmune disorders, and damaged tissue. These therapies are becoming more effective and are being used more frequently, but they are also becoming more complex. As a result, quality testing is becoming an increasingly important part of cellular therapy. Cellular therapies should be tested at several points during their production. The starting material, intermediate products and the final product are usually analyzed. Products are evaluated at critical steps in the manufacturing process and at the end of production prior to the release of the product for clinical use. In addition, the donor of the starting biologic material is usually evaluated. The testing of cellular therapies for stability, consistency, comparability and potency is especially challenging. We and others have found that global gene and microRNA expression analysis is useful for comparability testing and will likely be useful for potency, stability and consistency testing. Several examples of the use of gene expression analysis for assessing cellular therapies are presented.
Keyword
MeSH Terms
Figure
Reference
-
1. Fowler DH, Odom J, Steinberg SM, et al. Phase I clinical trial of costimulated, IL-4 polarized donor CD4+ T cells as augmentation of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006; 12:1150–1160. PMID: 17085308.
Article2. Mielke S, Solomon SR, Barrett AJ. Selective depletion strategies in allogeneic stem cell transplantation. Cytotherapy. 2005; 7:109–115. PMID: 16040390.
Article3. Rosenberg SA, Yannelli JR, Yang JC, et al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994; 86:1159–1166. PMID: 8028037.
Article4. Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009; 21:233–240. PMID: 19304471.
Article5. Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005; 105:3051–3057. PMID: 15632206.
Article6. Grzywacz B, Miller JS, Verneris MR. Use of natural killer cells as immunotherapy for leukaemia. Best Pract Res Clin Haematol. 2008; 21:467–483. PMID: 18790450.
Article7. Berg M, Lundqvist A, McCoy P Jr., et alClinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy. 2009; 11:341–355. PMID: 19308771.
Article8. Srivastava S, Lundqvist A, Childs RW. Natural killer cell immunotherapy for cancer: a new hope. Cytotherapy. 2008; 10:775–783. PMID: 19089686.
Article9. Guildline on potency testing of cell based immunotherapy medicinal products for the treatment of cancer. 2008. Accessed February 3, 2010. London: Committee for medicinal products for human use (CHMP);at http://www.ema.europa.eu/pdfs/human/cpwp/41086906enfin.pdf.10. Potency Measurements for Cell and Gene Therapy Products. 2006. In : Cellular, Tissue, and Gene Therapies Advisory Committee Meeting #41; US Food and Drug Administration.11. Specifications: test procedures and acceptance criteria for biotechnological/biological products. Q6B. International conference on harmonization of technical requirements for registration of pharmaceuticals for human use. 1999. Accessed February 3, 2010. at http://www.ich.org/LOB/media/MEDIA432.pdf.12. Howell WM, Rose-Zerilli MJ. Interleukin-10 polymorphisms, cancer susceptibility and prognosis. Fam Cancer. 2006; 5:143–149. PMID: 16736283.
Article13. Howell WM, Turner SJ, Bateman AC, Theaker JM. IL-10 promoter polymorphisms influence tumour development in cutaneous malignant melanoma. Genes Immun. 2001; 2:25–31. PMID: 11294564.
Article14. McCarron SL, Edwards S, Evans PR, et al. Influence of cytokine gene polymorphisms on the development of prostate cancer. Cancer Res. 2002; 62:3369–3372. PMID: 12067976.15. Hollegaard MV, Bidwell JL. Cytokine gene polymorphism in human disease: on-line databases, Supplement 3. Genes Immun. 2006; 7:269–276. PMID: 16642032.
Article16. Wang E, Miller LD, Ohnmacht GA, Liu ET, Marincola FM. High-fidelity mRNA amplification for gene profiling. Nat Biotechnol. 2000; 18:457–459. PMID: 10748532.
Article17. Dave SS, Fu K, Wright GW, et al. Molecular diagnosis of Burkitt's lymphoma. N Engl J Med. 2006; 354:2431–2442. PMID: 16760443.18. Halvorsen OJ, Oyan AM, Bø TH, et al. Gene expression profiles in prostate cancer: association with patient subgroups and tumour differentiation. Int J Oncol. 2005; 26:329–336. PMID: 15645116.
Article19. Wang E, Ngalame Y, Panelli MC, et al. Peritoneal and subperitoneal stroma may facilitate regional spread of ovarian cancer. Clin Cancer Res. 2005; 11:113–122. PMID: 15671535.20. Taniwaki M, Daigo Y, Ishikawa N, et al. Gene expression profiles of small-cell lung cancers: molecular signatures of lung cancer. Int J Oncol. 2006; 29:567–575. PMID: 16865272.
Article21. Basil CF, Zhao Y, Zavaglia K, et al. Common cancer biomarkers. Cancer Res. 2006; 66:2953–2961. PMID: 16540643.
Article22. miRBase: the microRNA database. Accessed February 3, 2010. Manchester, UK: at http://www.mirbase.org/index.shtml.23. Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002; 12:735–739. PMID: 12007417.
Article24. Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004; 303:83–86. PMID: 14657504.
Article25. Georgantas RW 3rd, Hildreth R, Morisot S, et al. CD34+ hematopoietic stem-progenitor cell microRNA expression and function: a circuit diagram of differentiation control. Proc Natl Acad Sci U S A. 2007; 104:2750–2755. PMID: 17293455.
Article26. Jin P, Han TH, Ren J, et al. Molecular signatures of maturing dendritic cells: implications for testing the quality of dendritic cell therapies. J Transl Med. 2010; 8:4. PMID: 20078880.
Article27. Felli N, Fontana L, Pelosi E, et al. MicroRNAs 221 and 222 inhibit normal erythropoiesis and erythroleukemic cell growth via kit receptor down-modulation. Proc Natl Acad Sci USA. 2005; 102:18081–18086. PMID: 16330772.
Article28. Han J, Farnsworth RL, Tiwari JL, et al. Quality prediction of cell substrate using gene expression profiling. Genomics. 2006; 87:552–559. PMID: 16413166.
Article29. Player A, Wang Y, Bhattacharya B, Rao M, Puri RK, Kawasaki ES. Comparisons between transcriptional regulation and RNA expression in human embryonic stem cell lines. Stem Cells Dev. 2006; 15:315–323. PMID: 16846370.
Article30. Bhattacharya B, Cai J, Luo Y, et al. Comparison of the gene expression profile of undifferentiated human embryonic stem cell lines and differentiating embryoid bodies. BMC Dev Biol. 2005; 5:22. PMID: 16207381.
Article31. Ren J, Jin P, Wang E, Marincola FM, Stroncek DF. MicroRNA and gene expression patterns in the differentiation of human embryonic stem cells. J Transl Med. 2009; 7:20. PMID: 19309508.
Article32. Shin JW, Jin P, Fan Y, et al. Evaluation of gene expression profiles of immature dendritic cells prepared from peripheral blood mononuclear cells. Transfusion. 2008; 48:647–657. PMID: 18282241.
Article33. Han TH, Jin P, Ren J, Slezak S, Marincola FM, Stroncek DF. Evaluation of 3 clinical dendritic cell maturation protocols containing lipopolysaccharide and interferon-gamma. J Immunother. 2009; 32:399–407. PMID: 19342965.34. Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005; 5:296–306. PMID: 15803149.
Article35. Albert ML, Jegathesan M, Darnell RB. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat Immunol. 2001; 2:1010–1017. PMID: 11590405.
Article36. Dieu MC, Vanbervliet B, Vicari A, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998; 188:373–386. PMID: 9670049.
Article37. Mailliard RB, Wankowicz-Kalinska A, Cai Q, et al. alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004; 64:5934–5937. PMID: 15342370.39. Ten Brinke A, Karsten ML, Dieker MC, Zwaginga JJ, van Ham SM. The clinical grade maturation cocktail monophosphoryl lipid A plus IFNgamma generates monocyte-derived dendritic cells with the capacity to migrate and induce Th1 polarization. Vaccine. 2007; 25:7145–7152. PMID: 17719152.40. Snijders A, Kalinski P, Hilkens CM, Kapsenberg ML. High-level IL-12 production by human dendritic cells requires two signals. Int Immunol. 1998; 10:1593–1598. PMID: 9846688.
Article41. Zobywalski A, Javorovic M, Frankenberger B, et al. Generation of clinical grade dendritic cells with capacity to produce biologically active IL-12p70. J Transl Med. 2007; 5:18. PMID: 17430585.
Article42. Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004; 10:475–480. PMID: 15122249.
Article43. Jonuleit H, Kuhn U, Muller G, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997; 27:3135–3142. PMID: 9464798.
Article44. Nicolette CA, Healey D, Tcherepanova I, et al. Dendritic cells for active immunotherapy: optimizing design and manufacture in order to develop commercially and clinically viable products. Vaccine. 2007; 25(Suppl 2):B47–B60. PMID: 17669561.
Article45. Nakahara T, Moroi Y, Uchi H, Furue M. Differential role of MAPK signaling in human dendritic cell maturation and Th1/Th2 engagement. J Dermatol Sci. 2006; 42:1–11. PMID: 16352421.
Article47. Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol. 2000; 164:4507–4512. PMID: 10779751.
Article48. Tjonnfjord GE, Steen R, Evensen SA, Thorsby E, Egeland T. Characterization of CD34+ peripheral blood cells from healthy adults mobilized by recombinant human granulocyte colony-stimulating factor. Blood. 1994; 84:2795–2801. PMID: 7522643.
Article49. Devine SM, Flomenberg N, Vesole DH, et al. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin's lymphoma. J Clin Oncol. 2004; 22:1095–1102. PMID: 15020611.
Article50. Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005; 201:1307–1318. PMID: 15837815.
Article51. Liles WC, Broxmeyer HE, Rodger E, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003; 102:2728–2730. PMID: 12855591.
Article52. Deichmann M, Kronenwett R, Haas R. Expression of the human immunodeficiency virus type-1 coreceptors CXCR-4 (fusin, LESTR) and CKR-5 in CD34+ hematopoietic progenitor cells. Blood. 1997; 89:3522–3528. PMID: 9160656.
Article53. Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999; 283:845–848. PMID: 9933168.
Article54. Ponomaryov T, Peled A, Petit I, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000; 106:1331–1339. PMID: 11104786.
Article55. Jung Y, Wang J, Schneider A, et al. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone. 2006; 38:497–508. PMID: 16337237.
Article56. Nervi B, Link DC, Dipersio JF. Cytokines and hematopoietic stem cell mobilization. J Cell Biochem. 2006; 99:690–705. PMID: 16888804.
Article57. Donahue RE, Jin P, Bonifacino AC, et al. Plerixafor (AMD3100) and granulocyte colony-stimulating factor (G-CSF) mobilize different CD34+ cell populations based on global gene and micro RNA expression signatures. Blood. 2009; 114:2530–2541. PMID: 19602709.58. Ceppi M, Pereira PM, Dunand-Sauthier I, et al. MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc Natl Acad Sci U S A. 2009; 106:2735–2740. PMID: 19193853.
Article59. Wu H, Neilson JR, Kumar P, et al. miRNA profiling of naive, effector and memory CD8 T cells. PLoS One. 2007; 2:e1020. PMID: 17925868.60. Ju X, Li D, Shi Q, Hou H, Sun N, Shen B. Differential microRNA expression in childhood B-cell precursor acute lymphoblastic leukemia. Pediatr Hematol Oncol. 2009; 26:1–10. PMID: 19206004.
Article61. Pitchford SC, Furze RC, Jones CP, Wengner AM, Rankin SM. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009; 4:62–72. PMID: 19128793.
Article62. Jin P, Wang E, Ren J, et al. Differentiation of two types of mobilized peripheral blood stem cells by microRNA and cDNA expression analysis. J Transl Med. 2008; 6:39. PMID: 18647411.
Article63. Rettig MP, Ramirez P, Nervi B, Dipersio JF. CXCR4 and mobilization of hematopoietic precursors. Methods Enzymol. 2009; 460:57–90. PMID: 19446720.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Government Regulations on Cellular Therapy and Proposed Approach Related with These Products by KFDA
- General Perspectives for Molecular Nuclear Imaging
- Established and Emerging Therapies in Acute Spinal Cord Injury
- A Review of Molecular Markers of Mature Odontoblasts and Their Role in Dentin Repair and Regeneration Research
- Pre- and Post-Treatment Imaging of Primary Central Nervous System Tumors in the Molecular and Genetic Era