Korean J Hematol.
2012 Mar;47(1):17-27. 10.5045/kjh.2012.47.1.17.
Cellular immunotherapy using dendritic cells against multiple myeloma
- Affiliations
-
- 1Research Center for Cancer Immunotherapy, Chonnam National University Hwasun Hospital, Hwasun, Korea. drjejung@chonnam.ac.kr
- 2Department of Hematology-Oncology, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 3Vaxcell-Bio Therapeutics, Hwasun, Korea.
- KMID: 2251967
- DOI: http://doi.org/10.5045/kjh.2012.47.1.17
Abstract
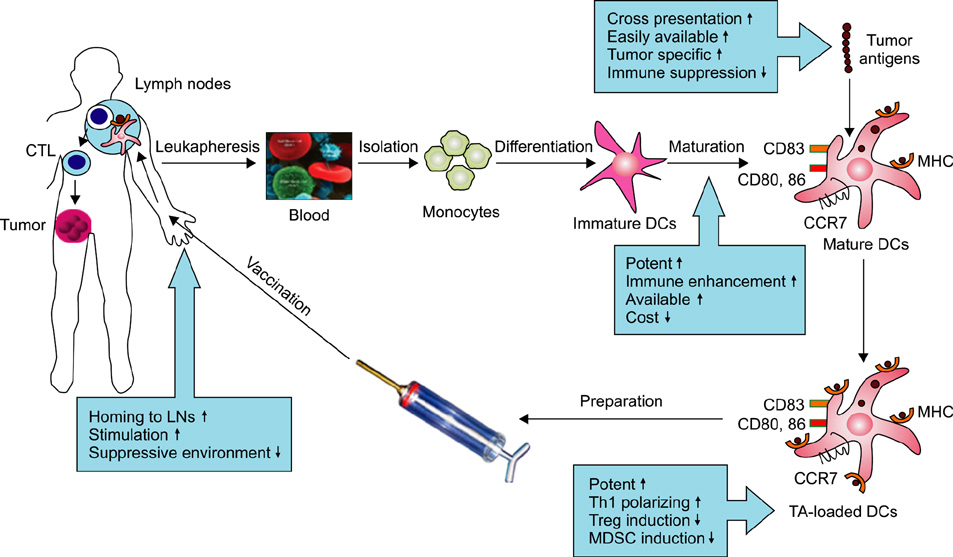
- Cellular therapy with dendritic cells (DCs) is emerging as a useful immunotherapeutic tool to treat multiple myeloma (MM). DC-based idiotype vaccination was recently suggested to induce idiotype-specific immune responses in MM patients. However, the clinical results so far have been largely disappointing, and the clinical effectiveness of such vaccinations in MM still needs to be demonstrated. DC-based therapies against MM may need to be boosted with other sources of tumor-associated antigens, and potent DCs should be recruited to increase the effectiveness of treatment. DCs with both high migratory capacity and high cytokine production are very important for effective DC-based cancer vaccination in order to induce high numbers of Th1-type CD4+ T cells and CD8+ cytotoxic T lymphocytes. The tumor microenvironment is also important in the regulation of tumor cell growth, proliferation, and the development of therapeutic resistance after treatment. In this review, we discuss how the efficacy of DC vaccination in MM can be improved. In addition, novel treatment strategies that target not only myeloma cells but also the tumor microenvironment are urgently needed to improve treatment outcomes.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Dendritic Cell-Based Cancer Immunotherapy against Multiple Myeloma: From Bench to Clinic
My-Dung Hoang, Sung-Hoon Jung, Hyun-Ju Lee, Youn-Kyung Lee, Thanh-Nhan Nguyen-Pham, Nu-Ri Choi, Manh-Cuong Vo, Seung-Shin Lee, Jae-Sook Ahn, Deok-Hwan Yang, Yeo-Kyeoung Kim, Hyeoung-Joon Kim, Je-Jung Lee
Chonnam Med J. 2015;51(1):1-7. doi: 10.4068/cmj.2015.51.1.1.Cellular immunotherapy as a beacon of hope for hematological malignancies
Hyun-Ju Lee, Sang-Ki Kim, Duck Cho, Je-Jung Lee
Blood Res. 2015;50(3):126-128. doi: 10.5045/br.2015.50.3.126.
Reference
-
1. Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004. 351:1860–1873.
Article2. Attal M, Harousseau JL. The role of high-dose therapy with autologous stem cell support in the era of novel agents. Semin Hematol. 2009. 46:127–132.
Article3. Perez-Simón JA, Martino R, Alegre A, et al. Chronic but not acute graft-versus-host disease improves outcome in multiple myeloma patients after non-myeloablative allogeneic transplantation. Br J Haematol. 2003. 121:104–108.
Article4. Harrison SJ, Cook G, Nibbs RJ, Prince HM. Immunotherapy of multiple myeloma: the start of a long and tortuous journey. Expert Rev Anticancer Ther. 2006. 6:1769–1785.
Article5. Tricot G, Vesole DH, Jagannath S, Hilton J, Munshi N, Barlogie B. Graft-versus-myeloma effect: proof of principle. Blood. 1996. 87:1196–1198.
Article6. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998. 392:245–252.
Article7. Reid DC. Dendritic cells and immunotherapy for malignant disease. Br J Haematol. 2001. 112:874–887.
Article8. Ridgway D. The first 1000 dendritic cell vaccinees. Cancer Invest. 2003. 21:873–886.
Article9. Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005. 5:296–306.
Article10. Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007. 121:1–14.
Article11. Quach H, Ritchie D, Stewart AK, et al. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia. 2010. 24:22–32.
Article12. Brown RD, Pope B, Murray A, et al. Dendritic cells from patients with myeloma are numerically normal but functionally defective as they fail to up-regulate CD80 (B7-1) expression after huCD40LT stimulation because of inhibition by transforming growth factor-beta1 and interleukin-10. Blood. 2001. 98:2992–2998.
Article13. Ratta M, Fagnoni F, Curti A, et al. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood. 2002. 100:230–237.
Article14. Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007. 7:41–51.
Article15. Ogawara H, Handa H, Yamazaki T, et al. High Th1/Th2 ratio in patients with multiple myeloma. Leuk Res. 2005. 29:135–140.
Article16. Maecker B, Anderson KS, von Bergwelt-Baildon MS, et al. Viral antigen-specific CD8+ T-cell responses are impaired in multiple myeloma. Br J Haematol. 2003. 121:842–848.
Article17. Dhodapkar MV, Geller MD, Chang DH, et al. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J Exp Med. 2003. 197:1667–1676.
Article18. Jarahian M, Watzl C, Issa Y, Altevogt P, Momburg F. Blockade of natural killer cell-mediated lysis by NCAM140 expressed on tumor cells. Int J Cancer. 2007. 120:2625–2634.
Article19. Prabhala RH, Neri P, Bae JE, et al. Dysfunctional T regulatory cells in multiple myeloma. Blood. 2006. 107:301–304.
Article20. Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar KM. Expansion of FOXP3 high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006. 108:2655–2661.
Article21. Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009. 182:4499–4506.
Article22. Brimnes MK, Vangsted AJ, Knudsen LM, et al. Increased level of both CD4+FOXP3+ regulatory T cells and CD14+HLA-DR-/low myeloid-derived suppressor cells and decreased level of dendritic cells in patients with multiple myeloma. Scand J Immunol. 2010. 72:540–547.
Article23. Kwak LW, Taub DD, Duffey PL, et al. Transfer of myeloma idiotype-specific immunity from an actively immunised marrow donor. Lancet. 1995. 345:1016–1020.
Article24. Bergenbrant S, Yi Q, Osterborg A, et al. Modulation of anti-idiotypic immune response by immunization with the autologous M-component protein in multiple myeloma patients. Br J Haematol. 1996. 92:840–846.
Article25. Li Y, Bendandi M, Deng Y, et al. Tumor-specific recognition of human myeloma cells by idiotype-induced CD8(+) T cells. Blood. 2000. 96:2828–2833.
Article26. Wen YJ, Barlogie B, Yi Q. Idiotype-specific cytotoxic T lymphocytes in multiple myeloma: evidence for their capacity to lyse autologous primary tumor cells. Blood. 2001. 97:1750–1755.
Article27. Butch AW, Kelly KA, Munshi NC. Dendritic cells derived from multiple myeloma patients efficiently internalize different classes of myeloma protein. Exp Hematol. 2001. 29:85–92.
Article28. Lim SH, Bailey-Wood R. Idiotypic protein-pulsed dendritic cell vaccination in multiple myeloma. Int J Cancer. 1999. 83:215–222.
Article29. Liso A, Stockerl-Goldstein KE, Auffermann-Gretzinger S, et al. Idiotype vaccination using dendritic cells after autologous peripheral blood progenitor cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2000. 6:621–627.
Article30. Titzer S, Christensen O, Manzke O, et al. Vaccination of multiple myeloma patients with idiotype-pulsed dendritic cells: immunological and clinical aspects. Br J Haematol. 2000. 108:805–816.
Article31. Yi Q, Desikan R, Barlogie B, Munshi N. Optimizing dendritic cell-based immunotherapy in multiple myeloma. Br J Haematol. 2002. 117:297–305.
Article32. Reichardt VL, Milazzo C, Brugger W, Einsele H, Kanz L, Brossart P. Idiotype vaccination of multiple myeloma patients using monocyte-derived dendritic cells. Haematologica. 2003. 88:1139–1149.33. Bendandi M, Rodríguez-Calvillo M, Inogés S, et al. Combined vaccination with idiotype-pulsed allogeneic dendritic cells and soluble protein idiotype for multiple myeloma patients relapsing after reduced-intensity conditioning allogeneic stem cell transplantation. Leuk Lymphoma. 2006. 47:29–37.
Article34. Yi Q, Szmania S, Freeman J, et al. Optimizing dendritic cell-based immunotherapy in multiple myeloma: intranodal injections of idiotype-pulsed CD40 ligand-matured vaccines led to induction of type-1 and cytotoxic T-cell immune responses in patients. Br J Haematol. 2010. 150:554–564.
Article35. Röllig C, Schmidt C, Bornhäuser M, Ehninger G, Schmitz M, Auffermann-Gretzinger S. Induction of cellular immune responses in patients with stage-I multiple myeloma after vaccination with autologous idiotype-pulsed dendritic cells. J Immunother. 2011. 34:100–106.
Article36. Brossart P, Schneider A, Dill P, et al. The epithelial tumor antigen MUC1 is expressed in hematological malignancies and is recognized by MUC1-specific cytotoxic T-lymphocytes. Cancer Res. 2001. 61:6846–6850.37. Milazzo C, Reichardt VL, Müller MR, Grünebach F, Brossart P. Induction of myeloma-specific cytotoxic T cells using dendritic cells transfected with tumor-derived RNA. Blood. 2003. 101:977–982.
Article38. Szmania S, Tricot G, van Rhee F. NY-ESO-1 immunotherapy for multiple myeloma. Leuk Lymphoma. 2006. 47:2037–2048.
Article39. Batchu RB, Moreno AM, Szmania SM, et al. Protein transduction of dendritic cells for NY-ESO-1-based immunotherapy of myeloma. Cancer Res. 2005. 65:10041–10049.
Article40. Chiriva-Internati M, Wang Z, Salati E, Bumm K, Barlogie B, Lim SH. Sperm protein 17 (Sp17) is a suitable target for immunotherapy of multiple myeloma. Blood. 2002. 100:961–965.
Article41. Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999. 10:673–679.
Article42. Ocadlikova D, Kryukov F, Mollova K, et al. Generation of myeloma-specific T cells using dendritic cells loaded with MUC1- and hTERT-drived nonapeptides or myeloma cell apoptotic bodies. Neoplasma. 2010. 57:455–464.
Article43. Qian J, Xie J, Hong S, et al. Dickkopf-1 (DKK1) is a widely expressed and potent tumor-associated antigen in multiple myeloma. Blood. 2007. 110:1587–1594.
Article44. Lee JJ, Choi BH, Kang HK, et al. Induction of multiple myeloma-specific cytotoxic T lymphocyte stimulation by dendritic cell pulsing with purified and optimized myeloma cell lysates. Leuk Lymphoma. 2007. 48:2022–2031.
Article45. Wen YJ, Min R, Tricot G, Barlogie B, Yi Q. Tumor lysate-specific cytotoxic T lymphocytes in multiple myeloma: promising effector cells for immunotherapy. Blood. 2002. 99:3280–3285.
Article46. Yang DH, Kim MH, Hong CY, et al. Alpha-type 1-polarized dendritic cells loaded with apoptotic allogeneic myeloma cell line induce strong CTL responses against autologous myeloma cells. Ann Hematol. 2010. 89:795–801.
Article47. Yang DH, Kim MH, Lee YK, et al. Successful cross-presentation of allogeneic myeloma cells by autologous alpha-type 1-polarized dendritic cells as an effective tumor antigen in myeloma patients with matched monoclonal immunoglobulins. Ann Hematol. 2011. 90:1419–1426.
Article48. Qian J, Wang S, Yang J, et al. Targeting heat shock proteins for immunotherapy in multiple myeloma: generation of myeloma-specific CTLs using dendritic cells pulsed with tumor-derived gp96. Clin Cancer Res. 2005. 11:8808–8815.
Article49. Qian J, Hong S, Wang S, et al. Myeloma cell line-derived, pooled heat shock proteins as a universal vaccine for immunotherapy of multiple myeloma. Blood. 2009. 114:3880–3889.
Article50. Hao S, Bi X, Xu S, et al. Enhanced antitumor immunity derived from a novel vaccine of fusion hybrid between dendritic and engineered myeloma cells. Exp Oncol. 2004. 26:300–306.51. Vasir B, Borges V, Wu Z, et al. Fusion of dendritic cells with multiple myeloma cells results in maturation and enhanced antigen presentation. Br J Haematol. 2005. 129:687–700.
Article52. Hayashi T, Hideshima T, Akiyama M, et al. Ex vivo induction of multiple myeloma-specific cytotoxic T lymphocytes. Blood. 2003. 102:1435–1442.
Article53. Rosenblatt J, Vasir B, Uhl L, et al. Vaccination with dendritic cell/tumor fusion cells results in cellular and humoral antitumor immune responses in patients with multiple myeloma. Blood. 2011. 117:393–402.
Article54. Lacy MQ, Mandrekar S, Dispenzieri A, et al. Idiotype-pulsed antigen-presenting cells following autologous transplantation for multiple myeloma may be associated with prolonged survival. Am J Hematol. 2009. 84:799–802.
Article55. Hsu FJ, Benike C, Fagnoni F, et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med. 1996. 2:52–58.
Article56. Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998. 4:328–332.
Article57. Madan RA, Gulley JL. Sipuleucel-T: harbinger of a new age of therapeutics for prostate cancer. Expert Rev Vaccines. 2011. 10:141–150.
Article58. Jonuleit H, Kühn U, Müller G, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997. 27:3135–3142.
Article59. Kalinski P, Vieira PL, Schuitemaker JH, de Jong EC, Kapsenberg ML. Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood. 2001. 97:3466–3469.
Article60. Yamazaki S, Inaba K, Tarbell KV, Steinman RM. Dendritic cells expand antigen-specific Foxp3+ CD25+ CD4+ regulatory T cells including suppressors of alloreactivity. Immunol Rev. 2006. 212:314–329.
Article61. de Vries IJ, Lesterhuis WJ, Scharenborg NM, et al. Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res. 2003. 9:5091–5100.62. Okada N, Iiyama S, Okada Y, et al. Immunological properties and vaccine efficacy of murine dendritic cells simultaneously expressing melanoma-associated antigen and interleukin-12. Cancer Gene Ther. 2005. 12:72–83.
Article63. Mailliard RB, Wankowicz-Kalinska A, Cai Q, et al. Alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004. 64:5934–5937.64. Lee JJ, Foon KA, Mailliard RB, Muthuswamy R, Kalinski P. Type 1-polarized dendritic cells loaded with autologous tumor are a potent immunogen against chronic lymphocytic leukemia. J Leukoc Biol. 2008. 84:319–325.
Article65. Wesa A, Kalinski P, Kirkwood JM, Tatsumi T, Storkus WJ. Polarized type-1 dendritic cells (DC1) producing high levels of IL-12 family members rescue patient TH1-type antimelanoma CD4+ T cell responses in vitro. J Immunother. 2007. 30:75–82.
Article66. Giermasz AS, Urban JA, Nakamura Y, et al. Type-1 polarized dendritic cells primed for high IL-12 production show enhanced activity as cancer vaccines. Cancer Immunol Immunother. 2009. 58:1329–1336.
Article67. Okada H, Kalinski P, Ueda R, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011. 29:330–336.
Article68. Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002. 195:327–333.
Article69. Mailliard RB, Son YI, Redlinger R, et al. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J Immunol. 2003. 171:2366–2373.
Article70. Pham TN, Hong CY, Min JJ, et al. Enhancement of antitumor effect using dendritic cells activated with natural killer cells in the presence of Toll-like receptor agonist. Exp Mol Med. 2010. 42:407–419.
Article71. Nguyen-Pham TN, Im CM, Nguyen TA, et al. Induction of myeloma-specific cytotoxic T lymphocytes responses by natural killer cells stimulated-dendritic cells in patients with multiple myeloma. Leuk Res. 2011. 35:1241–1247.
Article72. Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005. 6:769–776.
Article73. Frasca L, Fedele G, Deaglio S, et al. CD38 orchestrates migration, survival, and Th1 immune response of human mature dendritic cells. Blood. 2006. 107:2392–2399.
Article74. Faure-André G, Vargas P, Yuseff MI, et al. Regulation of dendritic cell migration by CD74, the MHC class II-associated invariant chain. Science. 2008. 322:1705–1710.
Article75. Nguyen-Pham TN, Lim MS, Nguyen TA, et al. Type I and II interferons enhance dendritic cell maturation and migration capacity by regulating CD38 and CD74 that have synergistic effects with TLR agonists. Cell Mol Immunol. 2011. 8:341–347.
Article76. Jung TY, Pham TN, Umeyama A, et al. Ursolic acid isolated from Uncaria rhynchophylla activates human dendritic cells via TLR2 and/or TLR4 and induces the production of IFN-gamma by CD4+ naive T cells. Eur J Pharmacol. 2010. 643:297–303.
Article77. Bae WK, Umeyama A, Chung IJ, Lee JJ, Takei M. Uncarinic acid C plus IFN-γ generates monocyte-derived dendritic cells and induces a potent Th1 polarization with capacity to migrate. Cell Immunol. 2010. 266:104–110.
Article78. Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002. 2:965–975.
Article79. Kitamura H, Kamon H, Sawa S, et al. IL-6-STAT3 controls intracellular MHC class II alphabeta dimer level through cathepsin S activity in dendritic cells. Immunity. 2005. 23:491–502.
Article80. Nefedova Y, Gabrilovich DI. Targeting of Jak/STAT pathway in antigen presenting cells in cancer. Curr Cancer Drug Targets. 2007. 7:71–77.
Article81. Wang S, Hong S, Yang J, et al. Optimizing immunotherapy in multiple myeloma: Restoring the function of patients' monocyte-derived dendritic cells by inhibiting p38 or activating MEK/ERK MAPK and neutralizing interleukin-6 in progenitor cells. Blood. 2006. 108:4071–4077.
Article82. Yang DH, Park JS, Jin CJ, et al. The dysfunction and abnormal signaling pathway of dendritic cells loaded by tumor antigen can be overcome by neutralizing VEGF in multiple myeloma. Leuk Res. 2009. 33:665–670.
Article83. Scandella E, Men Y, Gillessen S, Förster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002. 100:1354–1361.
Article84. Muthuswamy R, Urban J, Lee JJ, Reinhart TA, Bartlett D, Kalinski P. Ability of mature dendritic cells to interact with regulatory T cells is imprinted during maturation. Cancer Res. 2008. 68:5972–5978.
Article85. Liu JY, Wu Y, Zhang XS, et al. Single administration of low dose cyclophosphamide augments the antitumor effect of dendritic cell vaccine. Cancer Immunol Immunother. 2007. 56:1597–1604.
Article86. Höltl L, Ramoner R, Zelle-Rieser C, et al. Allogeneic dendritic cell vaccination against metastatic renal cell carcinoma with or without cyclophosphamide. Cancer Immunol Immunother. 2005. 54:663–670.
Article87. Galustian C, Meyer B, Labarthe MC, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother. 2009. 58:1033–1045.
Article88. Ramsay AG, Gribben JG. Immune dysfunction in chronic lymphocytic leukemia T cells and lenalidomide as an immunomodulatory drug. Haematologica. 2009. 94:1198–1202.
Article89. Cull G, Durrant L, Stainer C, Haynes A, Russell N. Generation of anti-idiotype immune responses following vaccination with idiotype-protein pulsed dendritic cells in myeloma. Br J Haematol. 1999. 107:648–655.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cellular immunotherapy in multiple myeloma
- Dendritic Cell-Based Cancer Immunotherapy against Multiple Myeloma: From Bench to Clinic
- Recent advances in cellular immunotherapy for lymphoid malignancies
- Immunotherapy using Dendritic Cells against Acute Myelogenous Leukemia
- Distinct features of dendritic cell-based immunotherapy as cancer vaccines