J Korean Acad Conserv Dent.
2011 Mar;36(2):139-148. 10.5395/JKACD.2011.36.2.139.
The effect of the strength and wetting characteristics of Bis-GMA/TEGDMA-based adhesives on the bond strength to dentin
- Affiliations
-
- 1Department of Conservative Dentistry, Seoul National University School of Dentistry and Dental Research Institute, Seoul, Korea. chobh@snu.ac.kr
- 2School of Chemical Engineering and Materials Science, Chung-Ang University, Seoul, Korea.
- 3Department of Conservative Dentistry, Section of Dentistry, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 2251819
- DOI: http://doi.org/10.5395/JKACD.2011.36.2.139
Abstract
OBJECTIVES
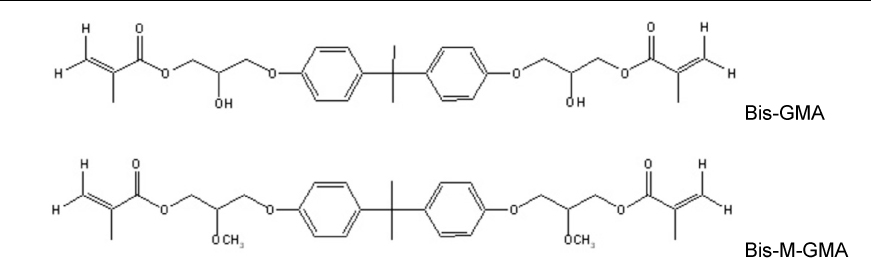
This study investigated the effect of the strength and wetting characteristics of adhesives on the bond strength to dentin. The experimental adhesives containing various ratios of hydrophobic, low-viscosity Bis-M-GMA, with Bis-GMA and TEGDMA, were made and evaluated on the mechanical properties and bond strength to dentin.
MATERIALS AND METHODS
Five experimental adhesives formulated with various Bis-GMA/Bis-M-GMA/TEGDMA ratios were evaluated on their viscosity, degree of conversion (DC), flexural strength (FS), and microtensile bond strength (MTBS). The bonded interfaces were evaluated with SEM and the solubility parameter was calculated to understand the wetting characteristics of the adhesives.
RESULTS
Although there were no significant differences in the DC between the experimental adhesives at 48 hr after curing (p > 0.05), the experimental adhesives that did not contain Bis-GMA exhibited a lower FS than did those containing Bis-GMA (p < 0.05). The experimental adhesives that had very little to no TEGDMA showed significantly lower MTBS than did those containing a higher content of TEGDMA (p < 0.05). The formers exhibited gaps at the interface between the adhesive layer and the hybrid layer. The solubility parameter of TEGDMA approximated those of the components of the primed dentin, rather than Bis-GMA and Bis-M-GMA.
CONCLUSIONS
To achieve a good dentin bond, a strong base monomer, such as Bis-GMA, cannot be completely replaced by Bis-M-GMA for maintaining mechanical strength. For compatible copolymerization between the adhesive and the primed dentin as well as dense cross-linking of the adhesive layer, at least 30% fraction of TEGDMA is also needed.
Keyword
MeSH Terms
Figure
Reference
-
1. Schwartz RS, Summitt JB, Robbins JW. Fundamentals of operative dentistry. 1996. 1st ed. Chicago: Quintessence Publishing Co;162–167.2. Van Meerbeek B, Willems G, Celis JP, Roos JR, Braem M, Lambrechts P, Vanherle G. Assessment by nano-indentation of the hardness and elasticity of the resin-dentin bonding area. J Dent Res. 1993. 72:1434–1442.
Article3. Dickens SH, Cho BH. Interpretation of bond failure through conversion and residual solvent measurements and Weibull analyses of flexural and microtensile bond strengths of bonding agents. Dent Mater. 2005. 21:354–364.
Article4. Bae JH, Cho BH, Kim JS, Kim MS, Lee IB, Son HH, Um CM, Kim CK, Kim OY. Adhesive layer properties as a determinant of dentin bond strength. J Biomed Mater Res B Appl Biomater. 2005. 74:822–828.
Article5. Miyazaki M, Ando S, Hinoura K, Onose H, Moore BK. Influence of filler addition to bonding agents on shear bond strength to bovine dentin. Dent Mater. 1995. 11:234–238.
Article6. Montes MA, de Goes MF, da Cunha MR, Soares AB. A morphological and tensile bond strength evaluation of an unfilled adhesive with low-viscosity composites and a filled adhesive in one and two coats. J Dent. 2001. 29:435–441.
Article7. Conde MC, Zanchi CH, Rodrigues-Junior SA, Carreno NL, Ogliari FA, Piva E. Nanofiller loading level: influence on selected properties of an adhesive resin. J Dent. 2009. 37:331–335.
Article8. Ferracane JL, Greener EH. The effect of resin formulation on the degree of conversion and mechanical properties of dental restorative resins. J Biomed Mater Res. 1986. 20:121–131.
Article9. Cadenaro M, Breschi L, Antoniolli F, Navarra CO, Mazzoni A, Tay FR, Di Lenarda R, Pashley DH. Degree of conversion of resin blends in relation to ethanol content and hydrophilicity. Dent Mater. 2008. 24:1194–1200.
Article10. Ogliari FA, Ely C, Lima GS, Conde MC, Petzhold CL, Demarco FF, Piva E. Onium salt reduces the inhibitory polymerization effect from an organic solvent in a model dental adhesive resin. J Biomed Mater Res B Appl Biomater. 2008. 86:113–118.
Article11. Guo X, Wang Y, Spencer P, Ye Q, Yao X. Effects of water content and initiator composition on photopolymerization of a model BisGMA/HEMA resin. Dent Mater. 2008. 24:824–831.
Article12. Malacarne J, Carvalho RM, de Goes MF, Svizero N, Pashley DH, Tay FR, Yiu CK, Carrilho MR. Water sorption/solubility of dental adhesive resins. Dent Mater. 2006. 22:973–980.
Article13. Peutzfeldt A. Resin composites in dentistry: the monomer systems. Eur J Oral Sci. 1997. 105:97–116.
Article14. Kim JW, Kim LU, Kim CK, Cho BH, Kim OY. Characteristics of novel dental composites containing 2,2-Bis[4-(2-methoxy-3-methacryloyloxy propoxy) phenyl] propane as a base resin. Biomacromolecules. 2006. 7:154–160.
Article15. Navarra CO, Cadenaro M, Armstrong SR, Jessop J, Antoniolli F, Sergo V, Di Lenarda R, Breschi L. Degree of conversion of Filtek Silorane Adhesive System and Clearfil SE Bond within the hybrid and adhesive layer: an in situ Raman analysis. Dent Mater. 2009. 25:1178–1185.
Article16. Ge J, Trujillo M, Stansbury J. Synthesis and photopolymerization of low shrinkage methacrylate monomers containing bulky substituent groups. Dent Mater. 2005. 21:1163–1169.
Article17. Rueggeberg FA, Hashinger DT, Fairhurst CW. Calibration of FTIR conversion analysis of contemporary dental resin composites. Dent Mater. 1990. 6:241–249.
Article18. Hoy KL. Tables of solubility parameters. Solvent and coatings materials research and development department. 1985. Union carbide corporation.19. Van Krevelen DW. Properties of polymers. 1990. 3rd ed. N.Y.: Elsevier science publishing co., Inc..20. Kalachandra S, Sankarapandian M, Shobha HK, Taylor DF, Mcgrath JE. Influence of hydrogen bonding on properties of Bis-GMA analogues. J Mater Sci Mater Med. 1997. 8:283–286.21. Pereira SG, Nunes TG, Kalachandra S. Low viscosity dimethacrylate comonomer compositions [Bis-GMA and CH3Bis-GMA] for novel dental composites; analysis of the network by stray-field MRI, solid-state NMR and DSC & FTIR. Biomaterials. 2002. 23:3799–3806.
Article22. Pereira SG, Osorio R, Toledano M, Nunes TG. Evaluation of two Bis-GMA analogues as potential monomer diluents to improve the mechanical properties of light-cured composite resins. Dent Mater. 2005. 21:823–830.
Article23. Tay FR, Pashley DH. Have dentin adhesives become too hydrophilic? J Can Dent Assoc. 2003. 69:726–731.24. Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, Rueggeberg FA, Foulger S, Saito T, Nishitani Y, Yoshiyama M, Tay FR, Pashley DH. Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials. 2005. 26:6449–6459.
Article25. Nishitani Y, Yoshiyama M, Donnelly AM, Agee KA, Sword J, Tay FR, Pashley DH. Effects of resin hydrophilicity on dentin bond strength. J Dent Res. 2006. 85:1016–1021.
Article26. Asmussen E. Restorative resins: hardness and strength vs. quantity of remaining double bonds. Scand J Dent Res. 1982. 90:484–489.
Article27. Seong SR, Seo DK, Lee IB, Son HH, Cho BH. Effect of exponential curing of composite resin on the microtensile dentin bond strength of adhesives. J Korean Acad Conserv Dent. 2010. 35:125–133.
Article28. Shin HJ, Song CK, Park SH, Kim JW, Cho KM. Physical properties of different self-adhesive resin cements and their shear bond strength on lithium disilicate ceramic and dentin. J Korean Acad Conserv Dent. 2009. 34:184–191.
Article29. Ko EJ, Shin DH. Difference in bond strength according to filling techniques and cavity walls in box-type occlusal composite resin restoration. J Korean Acad Conserv Dent. 2009. 34:350–356.
Article30. Erickson RL. Surface interactions of dentin adhesive materials. Oper Dent. 1992. Suppl 5. 81–94.31. Asmussen E, Uno S. Solubility parameters, fractional polarities, and bond strengths of some intermediary resins used in dentin bonding. J Dent Res. 1993. 72:558–565.
Article32. Miller RG, Bowles CQ, Chappelow CC, Eick JD. Application of solubility parameter theory to dentinbonding systems and adhesive strength correlations. J Biomed Mater Res. 1998. 41:237–243.
Article33. Hildebrand JH. The solubility of non-electrolytes. 1936. New York: Reinhold.34. Vaidyanathan TK, Vaidyanathan J. Recent advances in the theory and mechanism of adhesive resin bonding to dentin: a critical review. J Biomed Mater Res B Appl Biomater. 2009. 88:558–578.
Article35. Asmussen E, Hansen EK, Peutzfeldt A. Influence of the solubility parameter of intermediary resin on the effectiveness of the Gluma bonding system. J Dent Res. 1991. 70:1290–1293.
Article36. Finger WJ, Inoue M, Asmussen E. Effect of wettability of adhesive resins on bonding to dentin. Am J Dent. 1994. 7:35–38.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of hema and tegdma on the properties of experimental composite resins
- Effect of additional etching and ethanol-wet bonding on the dentin bond strength of one-step self-etch adhesives
- The effect of bonding resin on bond strength of dual-cure resin cements
- Bonding of a resin-modified glass ionomer cement to dentin using universal adhesives
- The effect of priming etched dentin with solvent on the microtensile bond strength of hydrophobic dentin adhesive