Impact of Immunohistochemistry-Based Molecular Subtype on Chemosensitivity and Survival in Patients with Breast Cancer Following Neoadjuvant Chemotherapy
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. drjiny@amc.seoul.kr
- 2Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2242191
- DOI: http://doi.org/10.4048/jbc.2012.15.2.203
Abstract
- PURPOSE
Pathologic complete response (pCR) has been suggested as a surrogate prognostic indicator in breast cancer patients treated with neoadjuvant chemotherapy. We assessed whether the likelihood of pCR and survival is associated with the immunohistochemistry-based molecular subtypes.
METHODS
We retrospectively analyzed the records of 276 patients with breast cancer who received neoadjuvant chemotherapy between January 2000 and January 2010. Patients were classified into four molecular subtypes based on the immunohistochemistry profiles of estrogen receptor, progesterone receptor, and HER2/neu. Logistic regression was used to analyze variables associated with pCR.
RESULTS
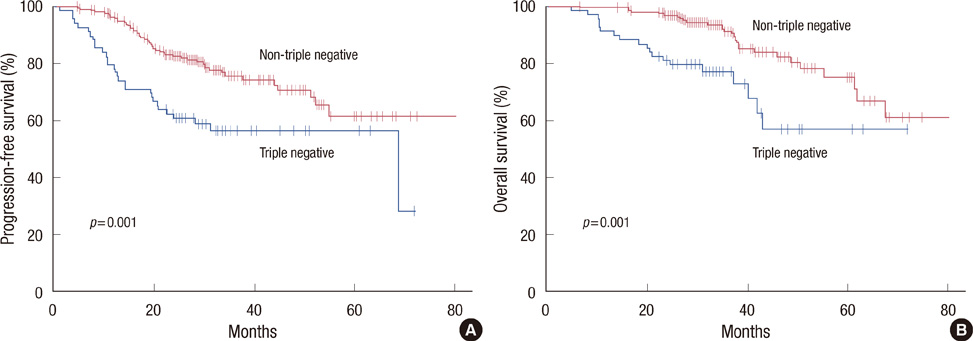
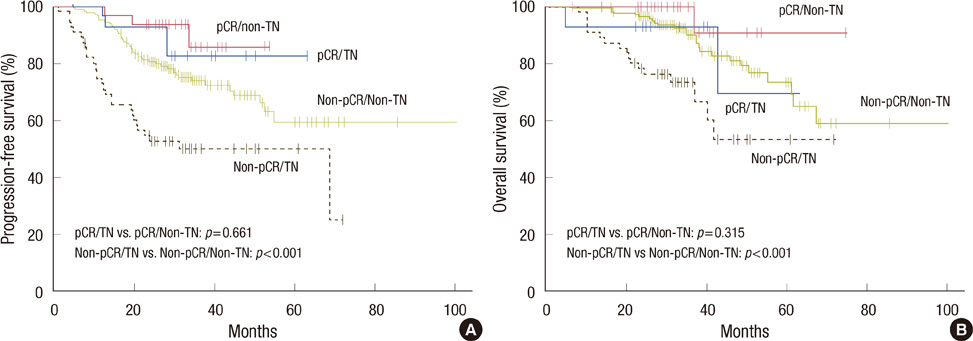
The pCR was achieved in 45 patients (16.3%). The triple negative subtype was an independent predictive factor for pCR (odds ratio, 3.21; 95% confidence interval, 1.20-8.56; p=0.020), and the ERBB-2 subtype showed a trend for higher pCR rates (odds ratio, 3.03; 95% confidence interval, 0.93-9.89; p=0.067) compared with the luminal A subtype. In 99 patients with HER2/neu-positive breast cancer, pCR rates were higher in those who received trastuzumab (31.7%) than those treated with conventional chemotherapy regimens (17.2%, p=0.023). The pCR was significantly associated with prolonged progression-free survival (p=0.008). The triple negative subgroup had shorter progression-free survival (p=0.001) and overall survival (p=0.001) than the other subgroups.
CONCLUSION
We demonstrated that the triple negative and ERBB-2 subtypes are more likely to obtain pCR when neoadjuvant chemotherapy is given, compared to the luminal A subtype. Despite the high pCR rate, the triple negative subtype showed worse survival outcomes, paradoxically, primarily due to patients who had residual disease.
MeSH Terms
-
Antibodies, Monoclonal, Humanized
Breast
Breast Neoplasms
Disease-Free Survival
Estrogens
Humans
Immunohistochemistry
Logistic Models
Neoadjuvant Therapy
Phenobarbital
Polymerase Chain Reaction
Receptors, Progesterone
Retrospective Studies
Trastuzumab
Antibodies, Monoclonal, Humanized
Estrogens
Phenobarbital
Receptors, Progesterone
Figure
Cited by 6 articles
-
Expression of Immunohistochemical Markers before and after Neoadjuvant Chemotherapy in Breast Carcinoma, and Their Use as Predictors of Response
Ho-chang Lee, Hyoungsuk Ko, Hyesil Seol, Dong-Young Noh, Wonshik Han, Tae-You Kim, Seock-Ah Im, In Ae Park
J Breast Cancer. 2013;16(4):395-403. doi: 10.4048/jbc.2013.16.4.395.Ki-67 as a Predictor of Response to Neoadjuvant Chemotherapy in Breast Cancer Patients
Kwan Il Kim, Kyung Hee Lee, Tae Ryung Kim, Yong Soon Chun, Tae Hoon Lee, Heung Kyu Park
J Breast Cancer. 2014;17(1):40-46. doi: 10.4048/jbc.2014.17.1.40.Sentinel Lymph Node Biopsy Alone after Neoadjuvant Chemotherapy in Patients with Initial Cytology-Proven Axillary Node Metastasis
Ji Young Kim, Min Kuk Kim, Jeong Eon Lee, Yongsik Jung, Soo Youn Bae, Se Kyung Lee, Won Ho Kil, Seok Won Kim, Ku Sang Kim, Seok Jin Nam, Sehwan Han
J Breast Cancer. 2015;18(1):22-28. doi: 10.4048/jbc.2015.18.1.22.Predictive and Prognostic Roles of Pathological Indicators for Patients with Breast Cancer on Neoadjuvant Chemotherapy
Xinyan Li, Mozhi Wang, Mengshen Wang, Xueting Yu, Jingyi Guo, Tie Sun, Litong Yao, Qiang Zhang, Yingying Xu
J Breast Cancer. 2019;22(4):497-521. doi: 10.4048/jbc.2019.22.e49.A Randomized Phase II Trial of Capecitabine Plus Vinorelbine Followed by Docetaxel Versus Adriamycin Plus Cyclophosphamide Followed by Docetaxel as Neoadjuvant Chemotherapy for Breast Cancer
Changhoon Yoo, Sung-Bae Kim, Jin-Hee Ahn, Jeong Eun Kim, Kyung Hae Jung, Gyung-Yub Gong, Byung-Ho Son, Sei-Hyun Ahn, Seung Do Ahn, Hak-Hee Kim, Hee Jung Shin, Woo Kun Kim
Cancer Res Treat. 2015;47(3):406-415. doi: 10.4143/crt.2014.073.Locoregional Recurrence by Tumor Biology in Breast Cancer Patients after Preoperative Chemotherapy and Breast Conservation Treatment
Eunjin Jwa, Kyung Hwan Shin, Ja Young Kim, Young Hee Park, So-Youn Jung, Eun Sook Lee, In Hae Park, Keun Seok Lee, Jungsil Ro, Yeon-Joo Kim, Tae Hyun Kim
Cancer Res Treat. 2016;48(4):1363-1372. doi: 10.4143/crt.2015.456.
Reference
-
1. Schwartz GF, Birchansky CA, Komarnicky LT, Mansfield CM, Cantor RI, Biermann WA, et al. Induction chemotherapy followed by breast conservation for locally advanced carcinoma of the breast. Cancer. 1994. 73:362–369.
Article2. Mauriac L, Durand M, Avril A, Dilhuydy JM. Effects of primary chemotherapy in conservative treatment of breast cancer patients with operable tumors larger than 3 cm. Results of a randomized trial in a single centre. Ann Oncol. 1991. 2:347–354.
Article3. van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001. 19:4224–4237.
Article4. Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008. 26:778–785.
Article5. Swain SM, Sorace RA, Bagley CS, Danforth DN Jr, Bader J, Wesley MN, et al. Neoadjuvant chemotherapy in the combined modality approach of locally advanced nonmetastatic breast cancer. Cancer Res. 1987. 47:3889–3894.6. Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999. 17:460–469.
Article7. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000. 406:747–752.
Article8. Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005. 11:5678–5685.
Article9. Goldstein NS, Decker D, Severson D, Schell S, Vicini F, Margolis J, et al. Molecular classification system identifies invasive breast carcinoma patients who are most likely and those who are least likely to achieve a complete pathologic response after neoadjuvant chemotherapy. Cancer. 2007. 110:1687–1696.
Article10. Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007. 13:2329–2334.
Article11. Bhargava R, Beriwal S, Dabbs DJ, Ozbek U, Soran A, Johnson RR, et al. Immunohistochemical surrogate markers of breast cancer molecular classes predicts response to neoadjuvant chemotherapy: a single institutional experience with 359 cases. Cancer. 2010. 116:1431–1439.
Article12. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, et al. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011. 22:1736–1747.
Article13. Greene FL. American Joint Committee on Cancer. AJCC Cancer Staging Manual. 2002. 6th ed. New York: Springer-Verlag.14. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998. 11:155–168.15. Desmedt C, Ruíz-García E, André F. Gene expression predictors in breast cancer: current status, limitations and perspectives. Eur J Cancer. 2008. 44:2714–2720.
Article16. Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California Cancer Registry. Cancer. 2007. 109:1721–1728.
Article17. Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008. 26:1275–1281.
Article18. Ring AE, Smith IE, Ashley S, Fulford LG, Lakhani SR. Oestrogen receptor status, pathological complete response and prognosis in patients receiving neoadjuvant chemotherapy for early breast cancer. Br J Cancer. 2004. 91:2012–2017.
Article19. Semiglazov VF, Semiglazov VV, Dashyan GA, Ziltsova EK, Ivanov VG, Bozhok AA, et al. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer. 2007. 110:244–254.
Article20. Smith IE, Dowsett M, Ebbs SR, Dixon JM, Skene A, Blohmer JU, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol. 2005. 23:5108–5116.
Article21. Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010. 375:377–384.
Article22. Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010. 28:2024–2031.
Article23. Buzdar AU, Valero V, Ibrahim NK, Francis D, Broglio KR, Theriault RL, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007. 13:228–233.
Article24. Dawson SJ, Provenzano E, Caldas C. Triple negative breast cancers: clinical and prognostic implications. Eur J Cancer. 2009. 45:Suppl 1. 27–40.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation between the molecular subtype of breast cancer and the in vitro adenosine triphosphate-based chemosensitivity assay
- Prognostic Factors in Patients with Locally Advanced Breast Cancer Treated by Neoadjuvant Chemotherapy
- Chemotherapy Response Assay Test and Prognosis for Breast Cancer Patients Who Have Undergone Anthracycline- and Taxane-Based Chemotherapy
- Correlation between Tumor Response to Neoadjuvant Chemotherapy and Patient Outcome in Breast Cancer
- The different prognostic impact of age according to individual molecular subtypes in breast cancer