J Breast Cancer.
2012 Jun;15(2):141-147. 10.4048/jbc.2012.15.2.141.
Enhanced Radiosensitivity and Chemosensitivity of Breast Cancer Cells by 2-Deoxy-D-Glucose in Combination Therapy
- Affiliations
-
- 1Department of Medical Physics, Tabriz University of Medical Sciences School of Medicine, Tabriz, Iran. pirayeshej@gmail.com
- 2Department of Immunology, Tabriz University of Medical Sciences School of Medicine, Tabriz, Iran.
- KMID: 2242183
- DOI: http://doi.org/10.4048/jbc.2012.15.2.141
Abstract
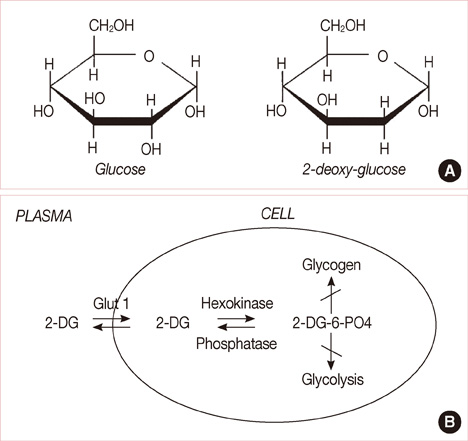
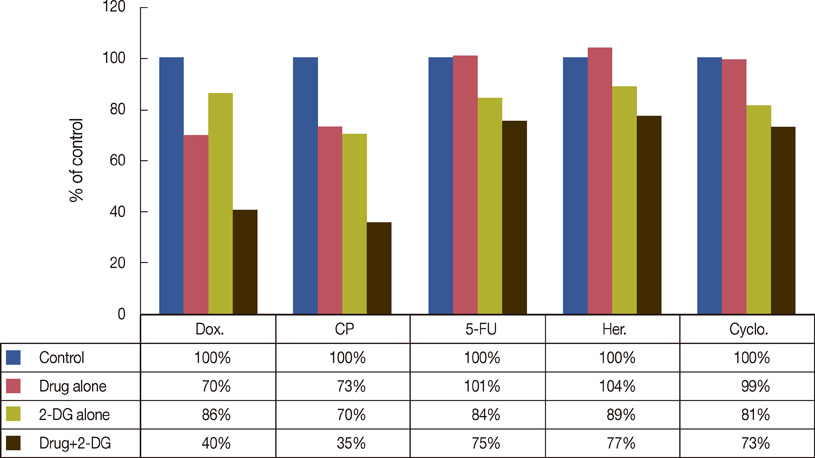
- Breast cancer is the most common malignancy, and it is also the major cause of cancer-related deaths of women worldwide. Breast cancer treatment involves surgery, chemotherapy, radiation therapy, or combination therapy, and novel strategies are needed to boost the oncologic outcome. The non-metabolizable glucose analogue, 2-deoxy-D-glucose (2-DG) which inhibits glucose synthesis and adenosine triphosphate production, is one of the important discoveries involving the disturbances that can be caused to the process of the metabolism. The glucose analogue, 2-DG, is known as a tumor sensitizer to irradiation (IR) and chemotherapy, which help improve the treatment rates. It enhances the cytotoxicity via oxidative stress, which is more redundant in tumor cells than in normal ones. This article provides a brief summary on studies related to 2-DG chemo-/radio-sensitization effects by combination therapy of 2-DG/IR or 2-DG/doxorubicin.
MeSH Terms
Figure
Reference
-
1. Agashe PP, Shrikhande SV. Genetics of breast cancer for the practicing surgeon. Accessed June 12th, 2011. http://www.bhj.org/journal/2001_4304_oct/review_537.htm.2. Elstner E, Williamson EA, Zang C, Fritz J, Heber D, Fenner M, et al. Novel therapeutic approach: ligands for PPARgamma and retinoid receptors induce apoptosis in bcl-2-positive human breast cancer cells. Breast Cancer Res Treat. 2002. 74:155–165.
Article3. Kaabinejadian S, Fouladdel SH, Ramezani M, Azizi E. p53 expression in MCF7, T47D and MDA-MB 468 breast cancer cell lines treated with adriamycin using RT-PCR and immunocytochemistry. J Biol Sci. 2008. 8:380–385.
Article4. de la Rochefordiere A, Asselain B, Campana F, Scholl SM, Fenton J, Vilcoq JR, et al. Age as prognostic factor in premenopausal breast carcinoma. Lancet. 1993. 341:1039–1043.
Article5. Han W, Kang SY. Korean Breast Cancer Society. Relationship between age at diagnosis and outcome of premenopausal breast cancer: age less than 35 years is a reasonable cut-off for defining young age-onset breast cancer. Breast Cancer Res Treat. 2010. 119:193–200.
Article6. Rodeh HJ, Eisel D, Frost I. Apoptosis, Cell Death and Cell Proliferation. 2000. 3rd ed. Mannheim: Roche Diagnostics GmbH;2–6.7. Types of breast cancer: ER positive, HER2 positive, and triple negative. WebMD. Accessed August 2nd, 2010. http://www.webmd.com/breast-cancer/breast-cancer-types-er-positive-her2-positive.8. Breast cancer: tumor biology. Winship Cancer Institute. Accessed August 4th, 2010. http://www.cancerquest.org/breast-cancer-molecular-biology.9. Cobb JP, Hotchkiss RS, Karl IE, Buchman TG. Mechanisms of cell injury and death. Br J Anaesth. 1996. 77:3–10.
Article10. Ghilotti M, Pierotti MA, Gariboldi M. Molecular markers for prediction of risk of radiation-related injury to normal tissue. J Nucleic Acids Investig. 2010. 1:55–61.
Article11. Dwarkanath BS, Zolzer F, Chandana S, Bauch T, Adhikari JS, Muller WU, et al. Heterogeneity in 2-deoxy-D-glucose-induced modifications in energetics and radiation responses of human tumor cell lines. Int J Radiat Oncol Biol Phys. 2001. 50:1051–1061.
Article12. Schwarz SB, Schaffer PM, Kulka U, Ertl-Wagner B, Hell R, Schaffer M. The effect of radio-adaptive doses on HT29 and GM637 cells. Radiat Oncol. 2008. 3:12.
Article13. Kunigal S, Lakka SS, Joseph P, Estes N, Rao JS. Matrix metalloproteinase-9 inhibition down-regulates radiation-induced nuclear factor-kappa B activity leading to apoptosis in breast tumors. Clin Cancer Res. 2008. 14:3617–3626.
Article14. Gupta S, Farooque A, Adhikari JS, Singh S, Dwarakanath BS. Enhancement of radiation and chemotherapeutic drug responses by 2-deoxy-D-glucose in animal tumors. J Cancer Res Ther. 2009. 5:Suppl 1. S16–S20.15. Mohanti BK, Rath GK, Anantha N, Kannan V, Das BS, Chandramouli BA, et al. Improving cancer radiotherapy with 2-deoxy-D-glucose: phase I/II clinical trials on human cerebral gliomas. Int J Radiat Oncol Biol Phys. 1996. 35:103–111.
Article16. Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006. 25:4633–4646.
Article17. Aft RL, Zhang FW, Gius D. Evaluation of 2-deoxy-D-glucose as a chemotherapeutic agent: mechanism of cell death. Br J Cancer. 2002. 87:805–812.
Article18. Maher JC, Krishan A, Lampidis TJ. Greater cell cycle inhibition and cytotoxicity induced by 2-deoxy-D-glucose in tumor cells treated under hypoxic vs aerobic conditions. Cancer Chemother Pharmacol. 2004. 53:116–122.
Article19. Sinthupibulyakit C, Grimes KR, Domann FE, Xu Y, Fang F, Ittarat W, et al. p53 is an important factor for the radiosensitization effect of 2-deoxy-D-glucose. Int J Oncol. 2009. 35:609–615.
Article20. Ahmad IM, Mustafa EH, Mustafa NH, Tahtamouni LH, Abdalla MY. 2DG enhances the susceptibility of breast cancer cells to doxorubicin. Cent Eur J Biol. 2010. 5:739–748.
Article21. Zhang F, Aft RL. Chemosensitizing and cytotoxic effects of 2-deoxy-D-glucose on breast cancer cells. J Cancer Res Ther. 2009. 5:Suppl 1. S41–S43.22. Kalia VK, Prabhakara S, Narayanan V. Modulation of cellular radiation responses by 2-deoxy-D-glucose and other glycolytic inhibitors: implications for cancer therapy. J Cancer Res Ther. 2009. 5:Suppl 1. S57–S60.
Article23. Dwarakanath BS. Cytotoxicity, radiosensitization, and chemosensitization of tumor cells by 2-deoxy-D-glucose in vitro. J Cancer Res Ther. 2009. 5:Suppl 1. S27–S31.24. Spitz DR, Sim JE, Ridnour LA, Galoforo SS, Lee YJ. Glucose deprivation-induced oxidative stress in human tumor cells. A fundamental defect in metabolism? Ann N Y Acad Sci. 2000. 899:349–362.
Article25. Aykin-Burns N, Ahmad IM, Zhu Y, Oberley LW, Spitz DR. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem J. 2009. 418:29–37.
Article26. Warburg O. On the origin of cancer cells. Science. 1956. 123:309–314.
Article27. Simons AL, Ahmad IM, Mattson DM, Dornfeld KJ, Spitz DR. 2-Deoxy-D-glucose combined with cisplatin enhances cytotoxicity via metabolic oxidative stress in human head and neck cancer cells. Cancer Res. 2007. 67:3364–3370.
Article28. Jain VK, Kalia VK, Sharma R, Maharajan V, Menon M. Effects of 2-deoxy-D-glucose on glycolysis, proliferation kinetics and radiation response of human cancer cells. Int J Radiat Oncol Biol Phys. 1985. 11:943–950.
Article29. Knowlton K, Mancini M, Creason S, Morales C, Hockenbery D, Anderson BO. Bcl-2 slows in vitro breast cancer growth despite its anti-apoptotic effect. J Surg Res. 1998. 76:22–26.
Article30. Butt AJ, Firth SM, King MA, Baxter RC. Insulin-like growth factor-binding protein-3 modulates expression of Bax and Bcl-2 and potentiates p53-independent radiation-induced apoptosis in human breast cancer cells. J Biol Chem. 2000. 275:39174–39181.
Article31. Ahmad IM, Abdalla MY, Aykin-Burns N, Simons AL, Oberley LW, Domann FE, et al. 2-Deoxyglucose combined with wild-type p53 overexpression enhances cytotoxicity in human prostate cancer cells via oxidative stress. Free Radic Biol Med. 2008. 44:826–834.
Article32. Singh G, Lakkis CL, Laucirica R, Epner DE. Regulation of prostate cancer cell division by glucose. J Cell Physiol. 1999. 180:431–438.
Article33. Zhao Y, Liu H, Liu Z, Ding Y, LeDoux SP, Wilson GL, et al. Overcoming trastuzumab resistance in breast cancer by targeting dysregulated glucose metabolism. Cancer Res. 2011. 71:4585–4597.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In Vitro Enhancement of Radiosensitivity by the Combination of Celecoxib and Ad-mda7 in Human Breast Cancer Cells
- Correlation between the molecular subtype of breast cancer and the in vitro adenosine triphosphate-based chemosensitivity assay
- Mono- and Combination Chemotherapy for Metastatic Breast Cancer: An Increamental Step Forward
- The Enhancement of Radiosensitivity by Celecoxib, Selective Cyclooxygenase-2 Inhibitor, on Human Cancer Cells Expressing Differential Levels of Cyclooxygenase-2
- The Reliability of Histoculture Drug Response Assay (HDRA) in Chemosensitivity Tests for Breast Cancer