Korean J Lab Med.
2006 Aug;26(4):263-268. 10.3343/kjlm.2006.26.4.263.
Evaluation of Plasma Homocysteine Assay by ADVIA Centaur Analyzer
- Affiliations
-
- 1Department of Laboratory Medicine, Sungkyunkwan University School of Medicine, Samsung Medical Center, Seoul, Korea. culture.kim@samsung.com
- KMID: 2238833
- DOI: http://doi.org/10.3343/kjlm.2006.26.4.263
Abstract
-
BACKGROUND: Hyperhomocysteinemia is a risk factor for the cardiovascular diseases that can be usually reversed with vitamin supplements. Quantitative analysis of homocysteine helps to identify patients who might get benefits from the intervention therapy. Recently, as the automated homocysteine assay (ADVIA Centaur, Bayer, USA) by direct chemiluminesence method has been developed, we evaluated the performance of the ADVIA Centaur immunoassay analyzer for homocysteine.
METHODS
The total plasma homocysteine concentrations were measured by ADVIA Centaur and by HPLC (HP1100 series with FLD, Hewlett Packard Co., Germany). To test the linearity, a dilution series was prepared. Between-run and total precision of the ADVIA Centaur assay were evaluated with Bio-Rad Homocysteine Controls for 10 days. The correlation was evaluated using 100 plasma samples from patients. NCCLS guidelines (EP5-A, EP6-P, and EP9-T) were followed to evaluate the ADVIA Centaur homocysteine assay.
RESULTS
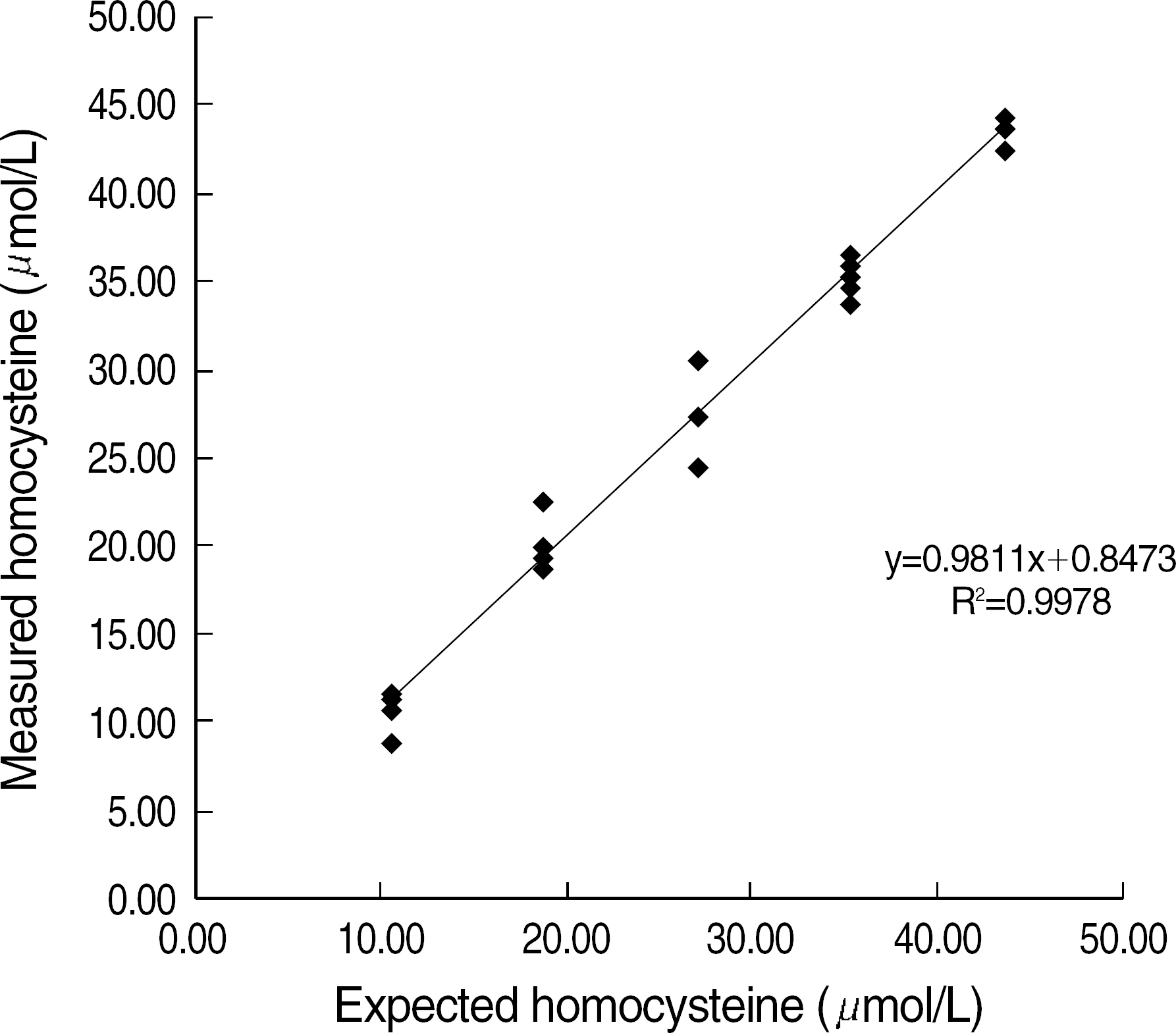
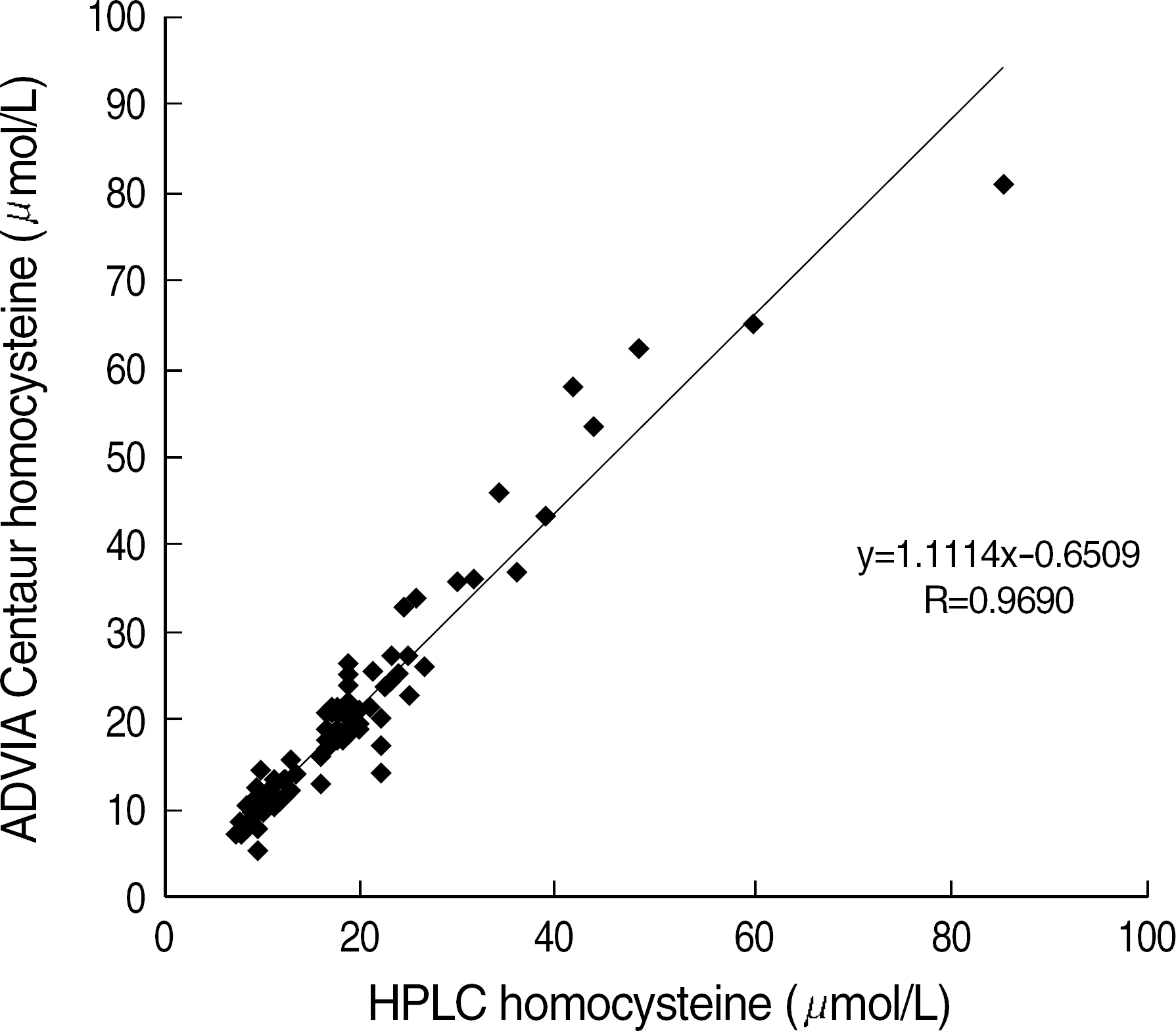
In the precision study, within-run and total run coefficients of variation (CV) of ADVIA Centaur assay were below 5%. The linearity was maintained well (R2=0.9978). The comparison study indicated a good correlation between the HPLC and ADVIA Centaur assay, and its correlation coefficient (R) was 0.9690.
CONCLUSIONS
Since the ADVIA Centaur homocysteine immunoassay showed an excellent correlation with the HPLC method and is more convenient, automatic, and rapid than the HPLC method, it should be potentially beneficial in the clinical laboratories.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, et al. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991; 324:1149–55.
Article2. Graham IM, Daly LE, Refsum HM, Robinson K, Brattstrom LE, Ueland PM, et al. Plasma homocysteine as a risk factor for vascular disease. The European Concerted Action Project. JAMA. 1997; 277:1775–81.
Article3. Perry IJ, Refsum H, Morris RW, Ebrahim SB, Ueland PM, Shaper AG. Prospective study of serum total homocysteine concentration and risk of stroke in middle-aged British men. Lancet. 1995; 346:1395–8.
Article4. Guba SC, Fink LM, Fonseca V. Hyperhomocysteinemia. An emerging and important risk factor for thromboembolic and cardiovascular disease. Am J Clin Pathol. 1996; 106:709–22.5. Alfthan G, Aro A, Gey KF. Plasma homocysteine and cardiovascular disease mortality. Lancet. 1997; 349:397.
Article6. Nygard O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med. 1997; 337:230–6.
Article7. Nilsson K, Gustafson L, Faldt R, Andersson A, Hultberg B. Plasma homocysteine in relation to serum cobalamin and blood folate in a phychogeriatric population. Eur J Clin Invest. 1994; 24:600–6.8. Palareti G, Salardi S, Piazzi S, Legnani C, Poggi M, Grauso F, et al. Blood coagulation changes in homocystinuria: effects of pyridoxine and other specific therapy. J Pediatr. 1986; 109:1001–6.
Article9. Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995; 274:1049–57.
Article10. Stampfer MJ, Malinow MR. Can lowering homocysteine levels reduce cardiovascular risk? N Engl J Med. 1995; 332:328–9.
Article11. Kang MS, Choi JW, Nahm CH, Lee JW, Lee CH, Kim JJ, et al. Comparison of high performance liquid chromatography and fluorescence polarization immunoassay for determination of total homocysteine in human plasma. Korean J Clin Pathol. 1999; 19:510–5.12. National Committe for Clinical Laboratory Standards. Evaluation of precision performance of clinical chemistry devices; Approved Guideline. NCCLS. Document EP5-A.Villanova, Pa: NCCLS;1999.13. National Committee for Clinical Laboratory Standards. Evaluation of the linearity of quantitative analytical methods; Proposed Guideline. NCLLS. Document EP6-P.Villanova, Pa.: NCCLS;1986.14. National Committe for Clinical Laboratory Standards. Method comparison and bias estimation using patient samples. Tentative guideline. Document EP9-T.Villanova, Pa.: NCCLS;1993.15. Andersson A, Isaksson A, Brattstrom L, Hultberg B. Homocysteine and other thiols determined in plasma by HPLC and thiol-specific postcolumn derivatization. Clin Chem. 1993; 39:1590–7.
Article16. Tewari PC, Zhang B, Bluestein BI. Analytical and clinical evaluation of the Bayer ADVIA Centaur homocysteine assay. Clin Chim Acta. 2004; 342:171–8.
Article17. Ueland PM, Refsum H, Stabler SP, Malinow MR, Andersson A, Allen RH. Total homocysteine in plasma or serum: methods and clinical applications. Clin Chem. 1993; 39:1764–79.
Article18. Hong KS, Chung IM. The Evaluation of the clinical significance and the normal reference range of the plasma homocysteine. Korean J Clin Pathol. 2000; 20:7–12.19. Fiskerstrand T, Refsum H, Kvalheim G, Ueland PM. Homocysteine and other thiols in plasma and urine: automated determination and sample stability. Clin Chem. 1993; 39:263–71.
Article20. Shipchandler MT, Moore EG. Rapid, fully automated measurement of plasma homocyst(e)ine with the Abbott IMx analyzer. Clin Chem. 1995; 41:991–4.
Article21. Kim HS, Lee YK, Lee KM, Jung JY. The measurement of homocyst(e)ine by IMx analyzer. J Lab Med Qual Assur. 1998; 20:411–5.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of ADVIA Centaur XPT
- Comparisons of Bayer ADVIA Centaur BNP Assay with Biosite Triage BNP Assay
- Evaluation of ADVIA Centaur HCV Assay for the Detection of Hepatitis C Virus Antibody: A Comparison Study with AxSYM HCV Version 3.0 Assay
- The Measurement of Homocyst(e)ine by IMx Analyzer
- Evaluation of the Dimension Vista 1500 Chemical Autoanalyzer