Clin Orthop Surg.
2015 Sep;7(3):383-391. 10.4055/cios.2015.7.3.383.
Demineralized Bone Matrix Injection in Consolidation Phase Enhances Bone Regeneration in Distraction Osteogenesis via Endochondral Bone Formation
- Affiliations
-
- 1Department of Orthopedic Surgery, Seoul National University Hospital, Seoul, Korea. leedy@snu.ac.kr
- KMID: 2234095
- DOI: http://doi.org/10.4055/cios.2015.7.3.383
Abstract
- BACKGROUND
Distraction osteogenesis (DO) is a promising tool for bone and tissue regeneration. However, prolonged healing time remains a major problem. Various materials including cells, cytokines, and growth factors have been used in an attempt to enhance bone formation. We examined the effect of percutaneous injection of demineralized bone matrix (DBM) during the consolidation phase on bone regeneration after distraction.
METHODS
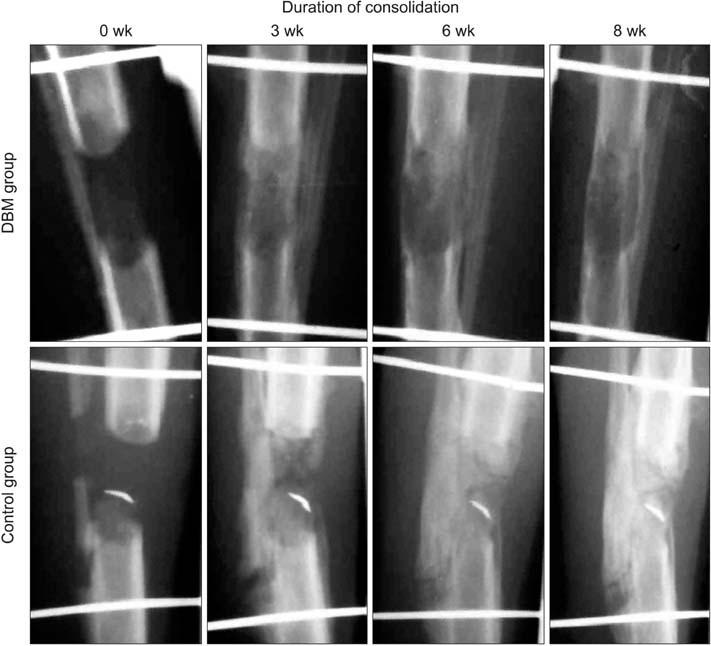
The immature rabbit tibial DO model (20 mm length-gain) was used. Twenty-eight animals received DBM 100 mg percutaneously at the end of distraction. Another 22 animals were left without further procedure (control). Plain radiographs were taken every week. Postmortem bone dual-energy X-ray absorptiometry and micro-computed tomography (micro-CT) studies were performed at the third and sixth weeks of the consolidation period and histological analysis was performed.
RESULTS
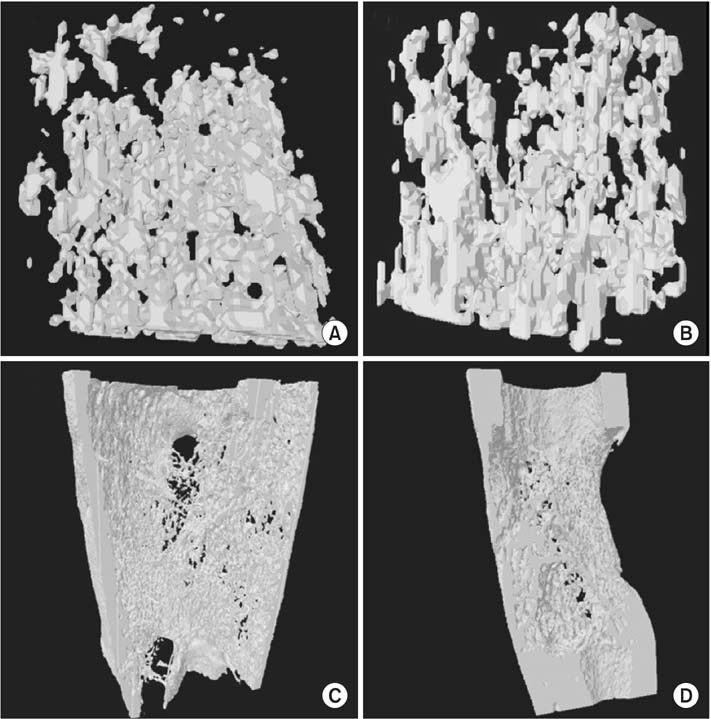
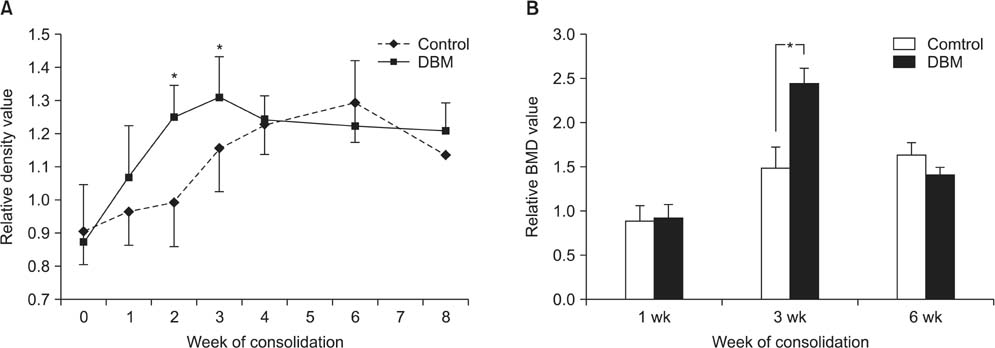
The regenerate bone mineral density was higher in the DBM group when compared with that in the saline injection control group at the third week postdistraction. Quantitative analysis using micro-CT revealed larger trabecular bone volume, higher trabecular number, and less trabecular separation in the DBM group than in the saline injection control group. Cross-sectional area and cortical thickness at the sixth week postdistraction, assessed using micro-CT, were greater in the regenerates of the DBM group compared with the control group. Histological evaluation revealed higher trabecular bone volume and trabecular number in the regenerate of the DBM group. New bone formation was apparently enhanced, via endochondral ossification, at the site and in the vicinity of the injected DBM. DBM was absorbed slowly, but it remained until the sixth postoperative week after injection.
CONCLUSIONS
DBM administration into the distraction gap at the end of the distraction period resulted in a significantly greater regenerate bone area, trabecular number, and cortical thickness in the rabbit tibial DO model. These data suggest that percutaneous DBM administration at the end of the distraction period or in the early consolidation period may stimulate regenerate bone formation and consolidation in a clinical situation with delayed bone healing during DO.
Keyword
MeSH Terms
Figure
Reference
-
1. Choi IH, Chung CY, Cho TJ, Yoo WJ. Angiogenesis and mineralization during distraction osteogenesis. J Korean Med Sci. 2002; 17(4):435–447.
Article2. Catagni MA, Guerreschi F, Holman JA, Cattaneo R. Distraction osteogenesis in the treatment of stiff hypertrophic nonunions using the Ilizarov apparatus. Clin Orthop Relat Res. 1994; (301):159–163.
Article3. Paley D. Treatment of tibial nonunion and bone loss with the Ilizarov technique. Instr Course Lect. 1990; 39:185–197.4. Moseley CF. Leg lengthening: a review of 30 years. Clin Orthop Relat Res. 1989; (247):38–43.5. Korzinek K, Tepic S, Perren SM. Limb lengthening and three-dimensional deformity corrections: a retrospective clinical study. Arch Orthop Trauma Surg. 1990; 109(6):334–340.
Article6. Fischgrund J, Paley D, Suter C. Variables affecting time to bone healing during limb lengthening. Clin Orthop Relat Res. 1994; (301):31–37.
Article7. Curran AR, Kuo KN, Lubicky JP. Simultaneous ipsilateral femoral and tibial lengthening with the Ilizarov method. J Pediatr Orthop. 1999; 19(3):386–390.
Article8. Noonan KJ, Leyes M, Forriol F, Canadell J. Distraction osteogenesis of the lower extremity with use of monolateral external fixation: a study of two hundred and sixty-one femora and tibiae. J Bone Joint Surg Am. 1998; 80(6):793–806.
Article9. Paley D. Problems, obstacles, and complications of limb lengthening by the Ilizarov technique. Clin Orthop Relat Res. 1990; (250):81–104.
Article10. Sailhan F. Bone lengthening (distraction osteogenesis): a literature review. Osteoporos Int. 2011; 22(6):2011–2015.
Article11. Kitoh H, Kitakoji T, Tsuchiya H, et al. Transplantation of marrow-derived mesenchymal stem cells and platelet-rich plasma during distraction osteogenesis: a preliminary result of three cases. Bone. 2004; 35(4):892–898.
Article12. Kitoh H, Kitakoji T, Tsuchiya H, Katoh M, Ishiguro N. Transplantation of culture expanded bone marrow cells and platelet rich plasma in distraction osteogenesis of the long bones. Bone. 2007; 40(2):522–528.
Article13. Zheng LW, Cheung LK. Effect of recombinant human bone morphogenetic protein-2 on mandibular distraction at different rates in a rabbit model. Tissue Eng. 2006; 12(11):3181–3188.
Article14. Li G, Bouxsein ML, Luppen C, et al. Bone consolidation is enhanced by rhBMP-2 in a rabbit model of distraction osteogenesis. J Orthop Res. 2002; 20(4):779–788.
Article15. Zakhary K, Motakis D, Hamdy RH, Campisi P, Amar Y, Lessard ML. Effect of recombinant human bone morphogenetic protein 7 on bone density during distraction osteogenesis of the rabbit mandible. J Otolaryngol. 2005; 34(6):407–414.
Article16. Okazaki H, Kurokawa T, Nakamura K, Matsushita T, Mamada K, Kawaguchi H. Stimulation of bone formation by recombinant fibroblast growth factor-2 in callotasis bone lengthening of rabbits. Calcif Tissue Int. 1999; 64(6):542–546.
Article17. Chang F, Mishima H, Ishii T, et al. Stimulation of EP4 receptor enhanced bone consolidation during distraction osteogenesis. J Orthop Res. 2007; 25(2):221–229.
Article18. Song HR, Oh CW, Kyung HS, et al. Injected calcium sulfate for consolidation of distraction osteogenesis in rabbit tibia. J Pediatr Orthop B. 2004; 13(3):170–175.
Article19. Van de Putte KA, Urist MR. Osteogenesis in the interior of intramuscular implants of decalcified bone matrix. Clin Orthop Relat Res. 1965; 43:257–270.
Article20. Urist MR. Bone: formation by autoinduction. Science. 1965; 150(3698):893–899.
Article21. Stevenson S. Enhancement of fracture healing with autogenous and allogeneic bone grafts. Clin Orthop Relat Res. 1998; 355 Suppl. S239–S246.
Article22. Zhang M, Powers RM Jr, Wolfinbarger L Jr. Effect(s) of the demineralization process on the osteoinductivity of demineralized bone matrix. J Periodontol. 1997; 68(11):1085–1092.
Article23. Gepstein R, Weiss RE, Hallel T. Bridging large defects in bone by demineralized bone matrix in the form of a powder: a radiographic, histological, and radioisotope-uptake study in rats. J Bone Joint Surg Am. 1987; 69(7):984–992.
Article24. Hagino T, Hamada Y. Accelerating bone formation and earlier healing after using demineralized bone matrix for limb lengthening in rabbits. J Orthop Res. 1999; 17(2):232–237.
Article25. Little DG, Smith NC, Williams PR, et al. Zoledronic acid prevents osteopenia and increases bone strength in a rabbit model of distraction osteogenesis. J Bone Miner Res. 2003; 18(7):1300–1307.
Article26. Ai-Aql ZS, Alagl AS, Graves DT, Gerstenfeld LC, Einhorn TA. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J Dent Res. 2008; 87(2):107–118.
Article27. Aronson J. Temporal and spatial increases in blood flow during distraction osteogenesis. Clin Orthop Relat Res. 1994; (301):124–131.
Article28. Aronson J, Good B, Stewart C, Harrison B, Harp J. Preliminary studies of mineralization during distraction osteogenesis. Clin Orthop Relat Res. 1990; (250):43–49.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Comparative Study on Regeneration of Bone Defects after the Grafts of Demineralized Bone Matrix and Hydroxyapatite
- Regeneration of Artificial Bone Defects by Allograft of Demineralized Bone and Bone Particles in Rabbits
- Effect of latency period and direction of distraction on the new bone formation during distraction osteogenesis
- The effect of oscillating distraction osteogenesis on new bone formation during mandibular distraction period in rabbits
- A histomorphometric study of bone formation around implants placed after vertical alveolar distraction in the dog medel