Korean Circ J.
2008 Jul;38(7):366-373. 10.4070/kcj.2008.38.7.366.
The Effects of Rosuvastatin on Plaque Regression in Patients Who Have a Mild to Moderate Degree of Coronary Stenosis With Vulnerable Plaque
- Affiliations
-
- 1The Heart Center of Chonnam National University Hospital, Cardiovascular Research Institute of Chonnam National University, Gwangju, Korea. myungho@chollian.net
- KMID: 2225770
- DOI: http://doi.org/10.4070/kcj.2008.38.7.366
Abstract
-
BACKGROUND AND OBJECTIVES: Intensive lipid-lowering therapy with statins improves the clinical outcomes and patient survival and it reduces the progression of atherosclerosis. Intravascular ultrasound (IVUS) has been used for calculating the plaque volumes to evaluate the mechanisms that may be involved in the progression or regression of coronary artery disease. We used serial IVUS exams to assess the efficacy of rosuvastatin on plaque regression in angina patients who had a mild to moderate degree of vulnerable plaque burden.
SUBJECTS AND METHODS
This study was a prospective, randomized, comparative study for lipid lowering therapy with using rosuvastatin 20 mg or atorvastatin 40 mg. IVUS was performed during the baseline coronary angiography and it was repeated after 12 months of treatment. The efficacy parameters included the changes in the atheroma volume and the lipid pool size as determined by IVUS. A total of 45 lesions in 30 patients were analyzed (rosuvastatin: 24 lesions in 16 patients vs. atorvastatin: 21 lesions in 14 patients).
RESULTS
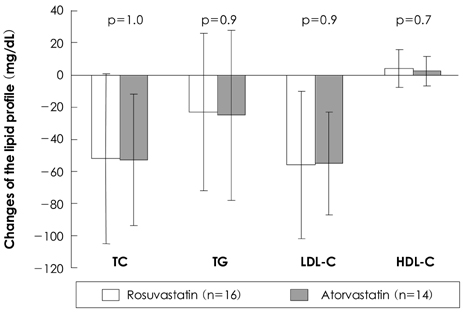
The low density lipoprotein (LDL)-cholesterol level was reduced from 121+/-45 mg/dL to 65+/-25 mg/dL in the rosuvastatin group (a 46% decrease, p<0.001), and from 127+/-37 mg/dL to 72+/-26 mg/dL in the atorvastatin group (a 43% decrease, p<0.001). The total atheroma and vessel volumes were significantly decreased, whereas the lumen volume was significantly increased from baseline to follow-up in both groups (for the rosuvastatin group: the total atheroma volume, 252+/-80 to 246+/-79 mm3, p<0.001; the vessel volume, 555+/-158 to 553+/-130 mm3, p<0.001; the lumen volume, 303+/-91 to 307+/-92 mm3, p<0.001, and for the atorvastatin group: the total atheroma volume, 288+/-98 to 283+/-98 mm3, p<0.001; the vessel volume, 607+/-165 to 604+/-166 mm3, p<0.001; the lumen volume, 319+/-71 to 321+/-73 mm3, p<0.001). The follow-up LDL-cholesterol level was correlated with the change in the total atheroma volume (r=0.577, p<0.001), the change in the percent atheroma volume (r=0.558, p<0.001) and the change in the lipid pool size (r=0.470, p=0.001).
CONCLUSION
Both rosuvastatin 20 mg and atorvastatin 40 mg could contribute to the regression of lipid-rich plaque. The follow-up LDL-cholesterol level is related to the regression and stabilization of vulnerable coronary plaque.
Keyword
MeSH Terms
-
Atherosclerosis
Coronary Angiography
Coronary Artery Disease
Coronary Stenosis
Fluorobenzenes
Follow-Up Studies
Glycosaminoglycans
Heptanoic Acids
Humans
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Lipoproteins
Plaque, Atherosclerotic
Prospective Studies
Pyrimidines
Pyrroles
Sulfonamides
Ultrasonics
Atorvastatin Calcium
Fluorobenzenes
Glycosaminoglycans
Heptanoic Acids
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Lipoproteins
Pyrimidines
Pyrroles
Sulfonamides
Figure
Reference
-
1. Scandinavian Simvastatin Survival Study Group. Randomized trial of cholesterol lowering in 4,444 patients with coronary heart disease. Lancet. 1994. 344:1383–1389.2. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995. 333:1301–1307.3. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996. 335:1001–1009.4. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998. 339:1349–1357.5. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. JAMA. 1998. 279:1615–1622.6. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002. 360:7–22.7. Hong YJ, Jeong MH, Lim JH, et al. The prognostic significance of statin therapy according to the level of C-reactive protein in acute myocardial infarction patients who underwent percutaneous coronary intervention. Korean Circ J. 2003. 33:891–900.8. Hong YJ, Jeong MH, Hwang SH, et al. Effect of combination therapy with simvastatin and carvedilol in patients with left ventricular dysfunction complicated with acute myocardial infarction who underwent percutaneous coronary intervention. Circ J. 2006. 70:1269–1274.9. Hong YJ, Jeong MH, Hyun DW, et al. Prognostic significance of simvastatin therapy in patients with ischemic heart failure who underwent percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol. 2005. 95:619–622.10. Falk E. Coronary thrombosis: pathogenesis and clinical manifestations. Am J Cardiol. 1991. 68:28B–35B.11. Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation. 2003. 108:1664–1672.12. Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS): a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001. 37:1478–1492.13. Sipahi I, Nicholls SJ, Tuzcu EM, Nissen SE. Coronary atherosclerosis can regress with very intensive statin therapy. Cleve Clin J Med. 2006. 73:937–944.14. Roberts WL, Moulton L, Law TC, et al. Evaluation of nine automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications: part 2. Clin Chem. 2001. 47:418–425.15. Strandberg TE, Vanhanen H, Tikkanen MJ. Effect of statins on C-reactive protein in patients with coronary artery disease. Lancet. 1999. 353:118–119.16. Ridker PM, Rifai N, Pfeffer MA, et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Circulation. 1998. 98:839–844.17. Park SY, Kwak JJ, Park SH. Dose dependent changes of lipid profiles, IL-6 and CRP in unstable angina patients after simvastatin therapy. Korean Circ J. 2003. 33:663–670.18. Dupuis J, Tardif JC, Cernacek P, Theroux P. Cholesterol reduction rapidly improves endothelial function after acute coronary syndromes. Circulation. 1999. 99:3227–3233.19. Son JW, Koh KK. Effects of statins on endothelium: vasomotor function, inflammation, and hemostasis. Korean Circ J. 1999. 29:1016–1031.20. Lacoste L, Lam JY, Hung J, Letchacovski G, Solymoss CB, Waters D. Hyperlipidemia and coronary disease: correction of the increased thrombogenic potential with cholesterol reduction. Circulation. 1995. 92:3172–3177.21. Kim YS, Ahn Y, Hong MH, et al. Rosuvastatin suppress the inflammatory responses through inhibition of c-Jun N-terminal kinase and nuclear factor-kappa B in endothelial cells. J Cardiovasc Pharmacol. 2007. 49:376–383.22. Nissen SE, Yock P. Intravascular ultrasound: novel pathophysiological insights and current clinical applications. Circulation. 2001. 103:604–616.23. Nissen SE. Effect of intensive lipid lowering on progression of coronary atherosclerosis: evidence for an early benefit from the Reversal of Atherosclerosis with Aggressive Lipid Lowering (REVERSAL) trial. Am J Cardiol. 2005. 96:61F–68F.24. Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis. JAMA. 2006. 295:1556–1565.25. Hong MK, Lee CW, Kim YH, et al. Usefulness of follow-up low-density lipoprotein cholesterol level as an independent predictor of changes of coronary atherosclerotic plaque size as determined by intravascular ultrasound analysis after statin (atorvastatin or simvastatin) therapy. Am J Cardiol. 2006. 98:866–870.26. Nicholls SJ, Tuzcu EM, Sipahi I, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA. 2007. 297:499–508.27. Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL-cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005. 352:29–38.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Plaque Characteristics on Coronary CT Angiography: Effects on the Degree of Coronary Artery Stenosis
- Statin Therapy with Coronary Plaque Imaging
- Acute coronary syndrome and vulnerable plaque
- F-18 Fluoride Positron Emission Tomography-Computed Tomography for Detecting Atherosclerotic Plaques
- Coronary Physiology-Based Approaches for Plaque Vulnerability: Implications for Risk Prediction and Treatment Strategies