Korean Circ J.
2008 Jul;38(7):360-365. 10.4070/kcj.2008.38.7.360.
Detection of Transferrin Receptor Over-Expression in a Rodent Model of Myocardial Ischemia/Reperfusion Injury Using Novel 99mTc Transferrin Conjugates: A Pilot Study
- Affiliations
-
- 1Department of Nuclear Medicine, Chonbuk National University Medical School, Jeonju, Korea. mhsohn@chonbuk.ac.kr
- 2Research Institute of Clinical Medicine, Chonbuk National University Medical School, Jeonju, Korea.
- 3Institute for Medical Sciences, Chonbuk National University Medical School, Jeonju, Korea.
- 4Department of Internal Medicine, Wonkwang University School of Medicine, Iksan, Korea.
- 5Diagnostic Pathology, Wonkwang University School of Medicine, Iksan, Korea.
- 6Department of Polymer Science and Engineering, Sunchon National University, Suncheon, Korea.
- KMID: 2225769
- DOI: http://doi.org/10.4070/kcj.2008.38.7.360
Abstract
-
BACKGROUND AND OBJECTIVES: Reperfusion of ischemic myocardium is necessary to salvage tissue from eventual death. However, new pathophysiological changes are initiated after reperfusion. The aim of this study was to investigate one of the mechanisms of ischemia/reperfusion (I/R) injury, and we focused on transferrin.
MATERIALS AND METHODS
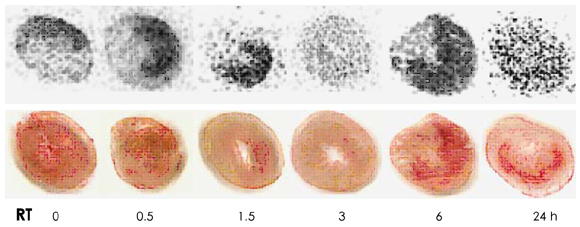
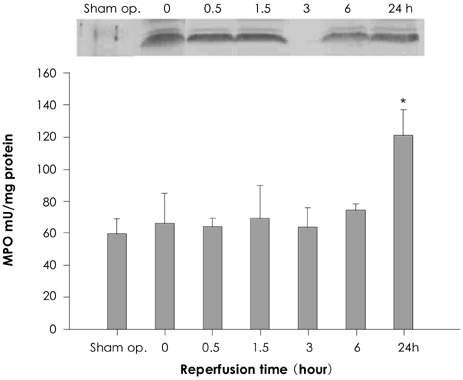
Male Spragre-Dawley (SD) rats were used for the I/R model. Myocardial ischemia was produced by occlusion of the left anterior descending coronary artery for 30 minutes. 99mTc Transferrin-Chitosan-hydrazino nicotinate hydrochloride (HYNIC) (Tfc) (/37 MBq/mL) was injected once after the reperfusion was finished. Autoradiography, hematoxylin and eosin (H & E) staining and determination of the tissue myeloperoxidase (MPO) activity were performed.
RESULTS
Autoradiography showed remarkable 99mTc-Tfc uptake in the left ventricular myocardium at the reperfusion period from 0 to 1.5 hours, whereas no uptake was demonstrated at 3 hours. The uptake was increased again at 6 and 24 hours. Western blotting showed that the transferrin receptor (TfR) proteins were increased at 0 to 1.5 hours compared with that of the control; this expression of TfR disappeared at 3 hours, and it showed up for the second time at 6 and 24 hours. The MPO activity only at 24 hours was significantly higher than that of the control and those MPO activities at 0 to 6 hours (p=0.001).
CONCLUSION
In the rodent model of 30 minutes occlusion and reperfusion, our study revealed, with using 99mTc-Tfc, that the TfR expression increased in the myocardium till 3 hours after reperfusion. TfR-mediated entry of iron into the cardiomyocytes may represent that this process plays a role in the I/R injury during the early reperfusion period.
Keyword
MeSH Terms
-
Animals
Autoradiography
Blotting, Western
Coronary Vessels
Eosine Yellowish-(YS)
Hematoxylin
Humans
Iron
Male
Myocardial Ischemia
Myocardium
Myocytes, Cardiac
Niacin
Peroxidase
Pilot Projects
Proteins
Rats
Receptors, Transferrin
Reperfusion
Reperfusion Injury
Rodentia
Transferrin
Eosine Yellowish-(YS)
Hematoxylin
Iron
Niacin
Peroxidase
Proteins
Receptors, Transferrin
Transferrin
Figure
Reference
-
1. Jaeschke H. Reactive oxygen and ischemia/reperfusion injury of the liver. Chem Biol Interact. 1991. 79:115–136.2. Kirschner RE, Fantini GA. Role of iron and oxygen-derived free radicals in ischemia-reperfusion injury. J Am Coll Surg. 1994. 179:103–117.3. Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000. 32:169–173.4. McCord JM. Effect of positive iron status at a cellular level. Nutr Rev. 1996. 54:85–88.5. Tacchini L, Fusar Poli D, Bernelli-Zazzera A, Cairo G. Transferrin receptor gene expression and transferrin-bound iron uptake are increased during postischemic rat liver reperfusion. Hepatology. 2002. 36:103–111.6. Omori N, Maruyama K, Jin G, et al. Targeting of post-ischemic cerebral endothelium in rat by liposomes bearing polyethylene glycol-coupled transferrin. Neurol Res. 2003. 25:275–279.7. Kim EM, Jeong HJ, Heo YJ, Moon HB, Bom HS, Kim CG. Intratumoral injection of 188Re labeled cationic polyethyleneimine conjugate: a preliminary report. J Korean Med Sci. 2004. 19:647–651.8. Kim EM, Jeong HJ, Kim SL, et al. Synthesis and in vivo evaluation of 99mTc-transferrin conjugate for detection of inflamed site. J Drug Target. 2007. 15:595–602.9. Lysiak JJ, Turner SD, Nguyen QA, Singbartl K, Ley K, Turner TT. Essential role of neutrophils in germ cell-specific apoptosis following ischemia/reperfusion injury of the mouse testis. Biol Reprod. 2001. 65:718–725.10. Taki J, Higuchi T, Kawashima A, et al. Detection of cardiomyocyte death in a rat model of ischemia and reperfusion using 99mTc-labeled annexin V. J Nucl Med. 2004. 45:1536–1541.11. Kim YK. Apoptosis in ischemia-reperfused myocardium of rabbit. Korean Circ J. 1997. 27:1017–1026.12. Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res. 2004. 61:481–497.13. de Vries B, Walter SJ, von Bonsdorff L, et al. Reduction of circulating redox-active iron by apotransferrin protects against renal ischemia-reperfusion injury. Transplantation. 2004. 77:669–675.14. Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004. 61:461–470.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of 99mTc-Transferrin as an Imaging Agent of Infectious Foci
- Transferrin Receptors in Gliomas and its Relationship with Flow Cytometric Analysis
- Transferrin receptor expression of the hyperplastic lesions of hepatocyte in experimental hepatocarcinogenesis
- Expression of Platelet Derived Growth Factor-A, C and Platelet Derived Growth Factor Receptor-alpha in the Ischemia Reperfusion Renal Failure Model
- Transferrin Analysis by Immunofixation for The Diagnosis of Cerebrospinal Fluid Leakage