Korean Circ J.
2013 Nov;43(11):744-751. 10.4070/kcj.2013.43.11.744.
Histopathological Comparison among Biolimus, Zotarolimus and Everolimus-Eluting Stents in Porcine Coronary Restenosis Model
- Affiliations
-
- 1Korea Cardiovascular Stent Institute, Jangseong, Korea. myungho@chollian.net
- 2Heart Research Center Nominated by Korea Ministry of Health and Welfare, Gwangju, Korea.
- 3Cardiovascular Research Center, Chonnam National University Hospital, Gwangju, Korea.
- 4Regeneromics Research Center, Chonnam National University, Gwangju, Korea.
- KMID: 2224806
- DOI: http://doi.org/10.4070/kcj.2013.43.11.744
Abstract
- BACKGROUND AND OBJECTIVES
The aim of this study was to examine the histolopathogical effects among the biolimus, zotarolimus, and everolimus eluting stent (EES) in the porcine coronary restenosis model.
SUBJECTS AND METHODS
Pigs were randomized into three groups in which the coronary arteries (15 pigs, 10 coronaries in each group) had either a biolimus A9 eluting stent (BES, n=10), zotarolimus eluting stent (ZES, n=10) or an EES (n=10). Histopathologic analysis was performed at 28 days after stenting.
RESULTS
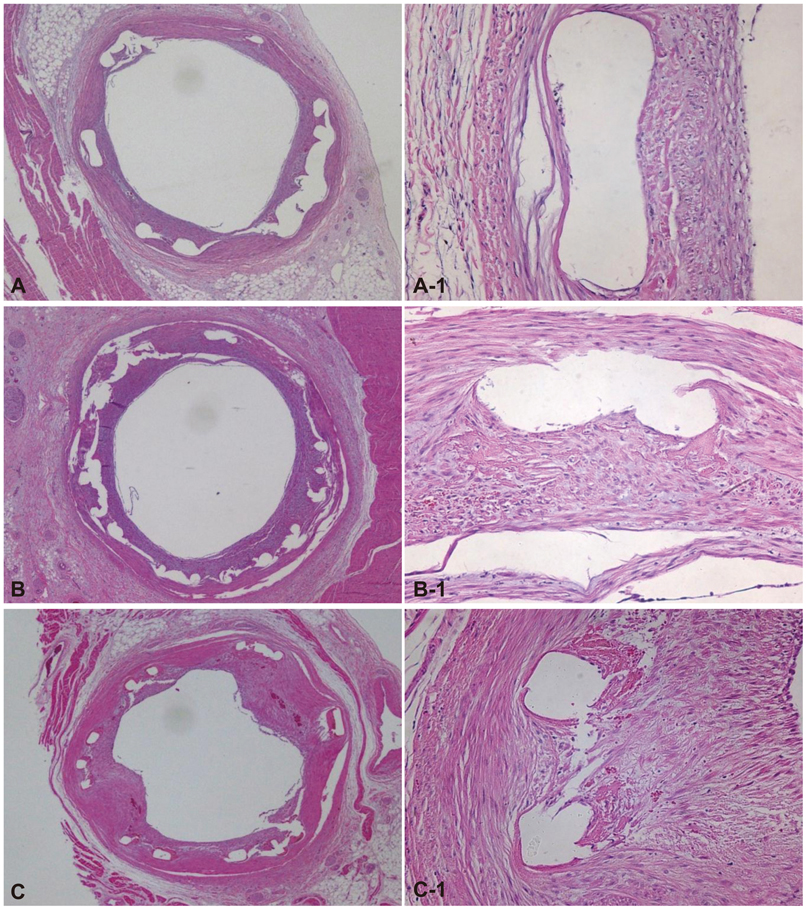
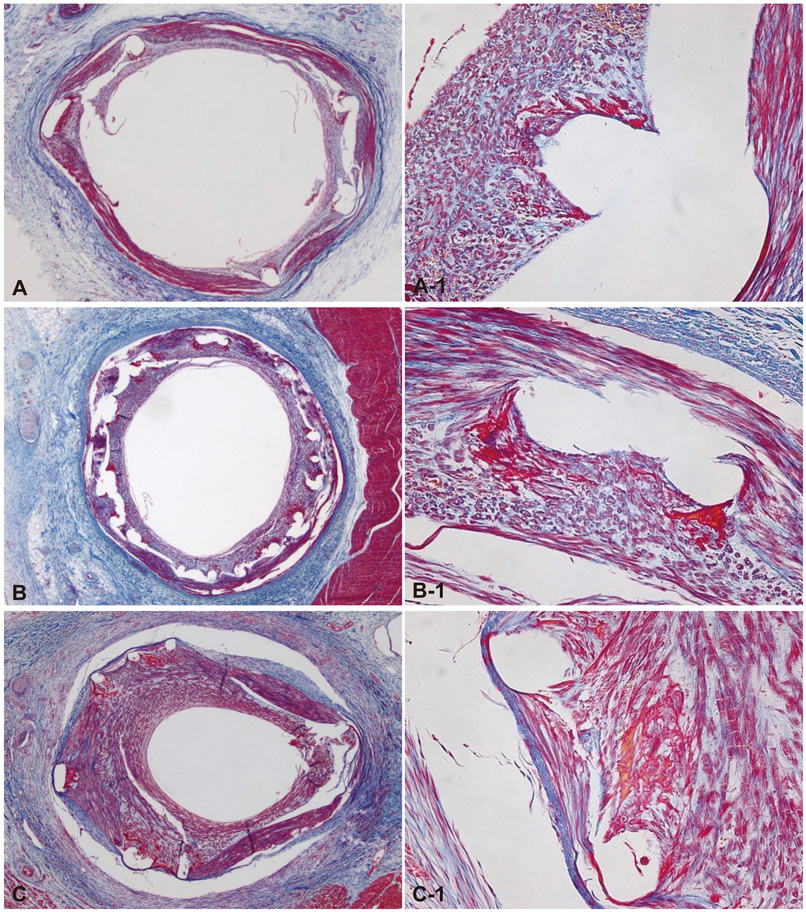
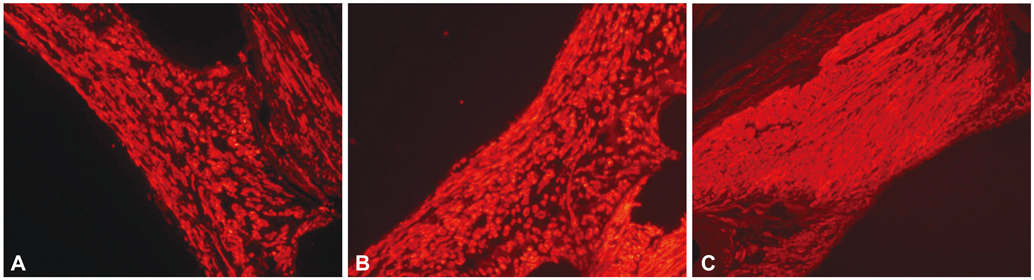
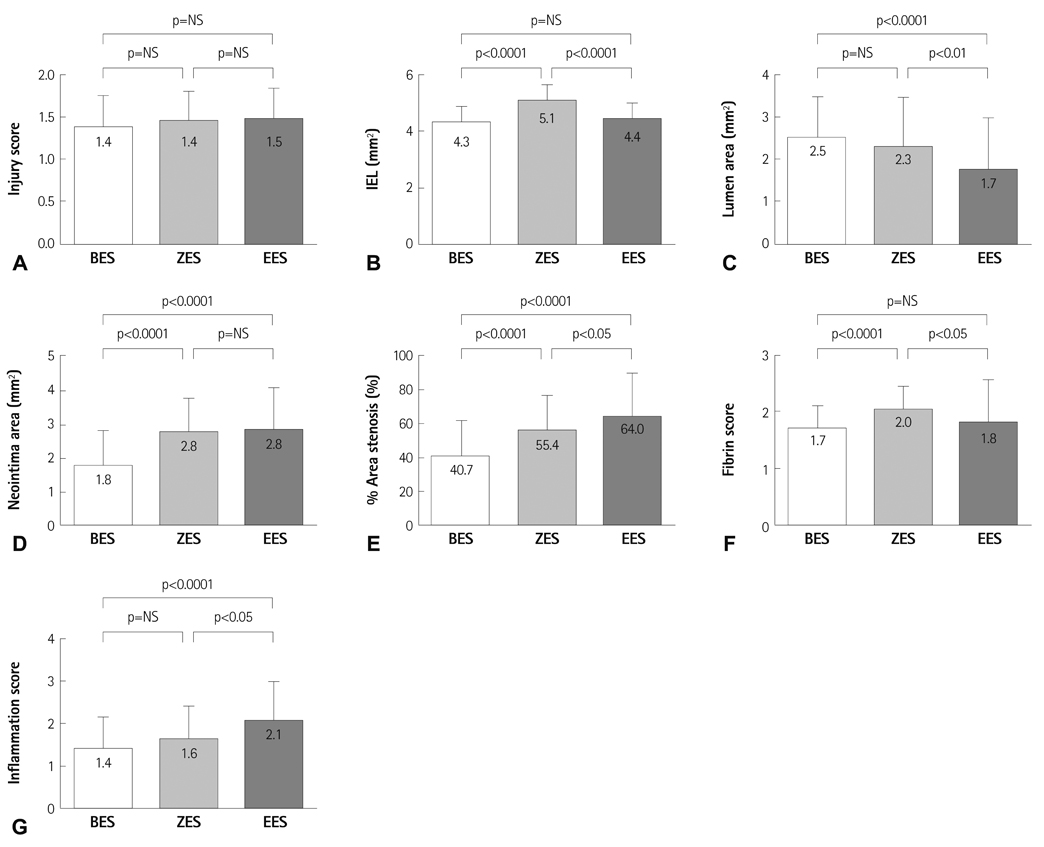
There were no significant differences in the injury score among the three groups. There was a significant difference in the internal elastic lamina, lumen area, neointima area, percent area stenosis, and the fibrin and inflammation score among the three groups (4.3+/-0.53 mm2, 2.5+/-0.93 mm2, 1.8+/-1.03 mm2, 40.7+/-20.80%, 1.7+/-0.41, 1.4+/-0.72 in the BES group vs. 5.1+/-0.55 mm2, 2.3+/-1.14 mm2, 2.8+/-1.00 mm2, 55.4+/-21.23%, 2.0+/-0.39, 1.6+/-0.76 in the ZES group vs. 4.4+/-0.53 mm2, 1.7+/-1.22 mm2, 2.8+/-1.23 mm2, 64.0+/-26.00%, 1.8+/-0.76, 2.1+/-0.90 in the EES group, respectively). BES is more effective in inhibiting neointimal hyperplasia compared to ZES and EES (p<0.0001). According to the fibrin and inflammation score, BES and EES are more effective in decreasing the fibrin deposition compared to ZES (p<0.001). Moreover, BES and ZES are more effective in reducing the inflammatory reaction compared to EES (p<0.001).
CONCLUSION
The result demonstrates that BES shows better histopathological characteristics than ZES and EES at one month after stenting in the porcine coronary restenosis model.
MeSH Terms
Figure
Cited by 1 articles
-
Effectiveness and Safety of Biolimus A9™-Eluting stEnt in Patients with AcUTe Coronary sYndrome; A Multicenter, Observational Study (BEAUTY Study)
Keun-Ho Park, Myung Ho Jeong, Young Joon Hong, Youngkeun Ahn, Hyun Kuk Kim, Young Yub Koh, Doo Il Kim, Sang Wook Kim, Weon Kim, Seung Woon Rha, Jay Young Rhew, Jong Seon Park, Hun Sik Park, Jang Ho Bae, Jang-Whan Bae, Seok Kyu Oh, Sung Yun Lee, Seung Wook Lee, Jae Hwan Lee, Sang Yeob Lim, Jang Hyun Cho, Kwang Soo Cha, Jai Keon Chae, Seung Ho Hur, Sun Ho Hwang, Jin Yong Hwang
Yonsei Med J. 2018;59(1):72-79. doi: 10.3349/ymj.2018.59.1.72.
Reference
-
1. Kushner FG, Hand M, Smith SC Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009; 54:2205–2241.2. Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Clinical outcomes with drug-eluting and bare metal stents in patients with ST-segment elevation myocardial infarction: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2013; 62:496–504.3. Min GS, Lee JH, Park JH, et al. Long-term safety and efficacy of sirolimus-and Paclitaxel-eluting stents in patients with acute myocardial infarction: four-year observational study. Korean Circ J. 2012; 42:266–273.4. Bangalore S, Kumar S, Fusaro M, et al. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation. 2012; 125:2873–2891.5. Jiménez-Quevedo P, Hernando L, Gómez-Hospital JA, et al. Sirolimuseluting stent versus bare metal stent in diabetic patients: the final fiveyear follow-up of the DIABETES trial. EuroIntervention. 2013; 9:328–335.6. De Luca G, Dirksen MT, Spaulding C, et al. Meta-analysis comparing efficacy and safety of first generation drug-eluting stents to bare-metal stents in patients with diabetes mellitus undergoing primary percutaneous coronary intervention. Am J Cardiol. 2013; 111:1295–1304.7. de Souza CF, El Mouallem AM, Brito Junior FS, et al. Safety and efficacy of biolimus-eluting stent with biodegradable polymer: insights from EINSTEIN (Evaluation of Next-generation drug-eluting STEnt IN patients with coronary artery disease) Registry. Einstein (Sao Paulo). 2013; 11:350–356.8. Kandzari DE, Leon MB, Meredith I, Fajadet J, Wijns W, Mauri L. Final 5-year outcomes from the endeavor zotarolimus-eluting stent clinical trial program: comparison of safety and efficacy with first-generation drug-eluting and bare-metal stents. JACC Cardiovasc Interv. 2013; 6:504–512.9. Sabate M, Cequier A, Iñiguez A, et al. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet. 2012; 380:1482–1490.10. Schwartz RS, Huber KC, Murphy JG, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol. 1992; 19:267–274.11. Jonas M, Edelman ER, Groothuis A, Baker AB, Seifert P, Rogers C. Vascular neointimal formation and signaling pathway activation in response to stent injury in insulin-resistant and diabetic animals. Circ Res. 2005; 97:725–733.12. Schwartz RS, Edelman E, Virmani R, et al. Drug-eluting stents in preclinical studies: updated consensus recommendations for preclinical evaluation. Circ Cardiovasc Interv. 2008; 1:143–153.13. Kolodgie FD, John M, Khurana C, et al. Sustained reduction of in-stent neointimal growth with the use of a novel systemic nanoparticle paclitaxel. Circulation. 2002; 106:1195–1198.14. Onuma Y, Miquel-Hebert K, Serruys PW. SPIRIT II Investigators. Five-year long-term clinical follow-up of the XIENCE V everolimus-eluting coronary stent system in the treatment of patients with de novo coronary artery disease: the SPIRIT II trial. EuroIntervention. 2013; 8:1047–1051.15. Yamasaki M, Tsujino I, Lima-Filho MO, et al. Comparison of vascular response to the everolimus-eluting stent versus the paclitaxel-eluting stent: intravascular ultrasound results from the SPIRIT III trial. EuroIntervention. 2012; 8:724–731.16. Vardi M, Burke DA, Bangalore S, et al. Long term efficacy and safety of zotarolimus-eluting stent in patients with diabetes mellitus: Pooled 5-year results from the ENDEAVOR III and IV trials. Catheter Cardiovasc Interv. 2013; [Epub ahead of print].17. Kim SJ, Lee H, Cho JM, et al. Comparison of zotarolimus-eluting stent and everolimus-eluting stent for vascular healing response: serial 3-month and 12-month optical coherence tomography study. Coron Artery Dis. 2013; 24:431–439.18. Park KW, Lee JM, Kang SH, et al. Safety and efficacy of second-generation everolimus-eluting Xience V stents versus zotarolimus-eluting resolute stents in real-world practice: patient-related and stent-related outcomes from the multicenter prospective EXCELLENT and RESOLUTE-Korea registries. J Am Coll Cardiol. 2013; 61:536–544.19. Hannan EL, Zhong Y, Wu C, et al. Everolimus-eluting stents and zotarolimus-eluting stents for percutaneous coronary interventions: two-year outcomes in New York State. Catheter Cardiovasc Interv. 2013; 81:1097–1105.20. Smits PC, Hofma S, Togni M, et al. Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent (COMPARE II): a randomised, controlled, non-inferiority trial. Lancet. 2013; 381:651–660.21. Natsuaki M, Kozuma K, Morimoto T, et al. Biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent: a randomized, controlled, noninferiority trial. J Am Coll Cardiol. 2013; 62:181–190.22. Stefanini GG, Byrne RA, Serruys PW, et al. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J. 2012; 33:1214–1222.23. Park KH, Jeong MH, Kim JM, et al. The impact of triple anti-platelet therapy for endothelialization and inflammatory response at overlapping bioabsorbable polymer coated drug-eluting stents in a porcine coronary model. Int J Cardiol. 2013; 168:1853–1858.24. Räber L, Kelbæk H, Ostoijc M, et al. Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA. 2012; 308:777–787.25. Vorpahl M, Yazdani SK, Nakano M, et al. Pathobiology of stent thrombosis after drug-eluting stent implantation. Curr Pharm Des. 2010; 16:4064–4071.26. Byrne RA, Joner M, Kastrati A. Polymer coatings and delayed arterial healing following drug-eluting stent implantation. Minerva Cardioangiol. 2009; 57:567–584.27. Tada N, Virmani R, Grant G, et al. Polymer-free biolimus a9-coated stent demonstrates more sustained intimal inhibition, improved healing, and reduced inflammation compared with a polymer-coated sirolimus-eluting cypher stent in a porcine model. Circ Cardiovasc Interv. 2010; 3:174–183.28. Grube E, Buellesfeld L. BioMatrix Biolimus A9-eluting coronary stent: a next-generation drug-eluting stent for coronary artery disease. Expert Rev Med Devices. 2006; 3:731–741.29. Ostojic M, Sagic D, Jung R, et al. The pharmacokinetics of Biolimus A9 after elution from the Nobori stent in patients with coronary artery disease: the NOBORI PK study. Catheter Cardiovasc Interv. 2008; 72:901–908.30. Malik N, Gunn J, Holt CM, et al. Intravascular stents: a new technique for tissue processing for histology, immunohistochemistry, and transmission electron microscopy. Heart. 1998; 80:509–516.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Pretreatment of Ezetimibe/Simvastatin on Arterial Healing and Endothelialization after Drug-Eluting Stent Implantation in a Porcine Coronary Restenosis Model
- A Patient with Repeated Catastrophic Multi-Vessel Coronary Spasm after Zotarolimus-Eluting Stent Implantation
- Drug-Eluting Stent Strut Fracture as a Cause of Restenosis
- Preclinical Evaluation of a Novel Polymer-free Everolimus-eluting Stent in a Mid-term Porcine Coronary Restenosis Model
- Systemic drug therapy and restenosis after drug-eluting stent implantation