Korean Circ J.
2015 Mar;45(2):110-116. 10.4070/kcj.2015.45.2.110.
Effect of Pretreatment of Ezetimibe/Simvastatin on Arterial Healing and Endothelialization after Drug-Eluting Stent Implantation in a Porcine Coronary Restenosis Model
- Affiliations
-
- 1The Heart Research Center of Chonnam National University Hospital Designated by Korea Ministry of Health, Welfare and Family Affairs, Gwangju, Korea. myungho@chollian.net
- KMID: 2297877
- DOI: http://doi.org/10.4070/kcj.2015.45.2.110
Abstract
- BACKGROUND AND OBJECTIVES
We sought to evaluate the effect of the early use of ezetimibe/simvastatin (Vytorin(R)) on arterial healing and endothelialization after the implantation of a drug-eluting stent (DES) in a porcine model of coronary restenosis.
MATERIALS AND METHODS
A total of 20 pigs (40 coronary arteries) were randomly allocated to a pretreatment or no treatment group. The pretreatment group (n=20) received oral ezetimibe/simvastatin (10/20 mg) daily for 7 days before stenting and the no pretreatment group (n=20) did not. All pigs were treated with ezetimibe/simvastatin (10/20 mg) daily after stenting for 4 weeks. Stenting was performed using a bare-metal stent (BMS, n=10) and three types of DES: biolimus A9-eluting stent (BES, n=10), zotarolimus-eluting stent (ZES, n=10), and everolimus-eluting stents (EES, n=10). Four weeks later, pigs underwent a follow-up coronary angiography and were sacrificed for histopathologic analysis.
RESULTS
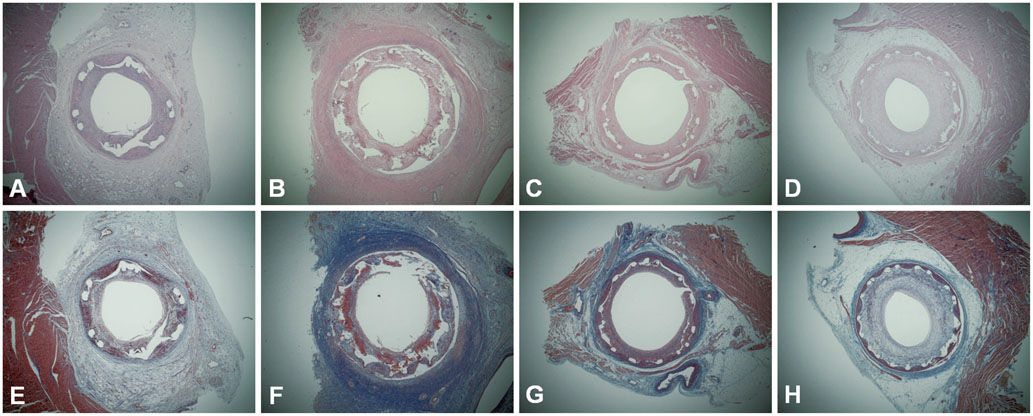
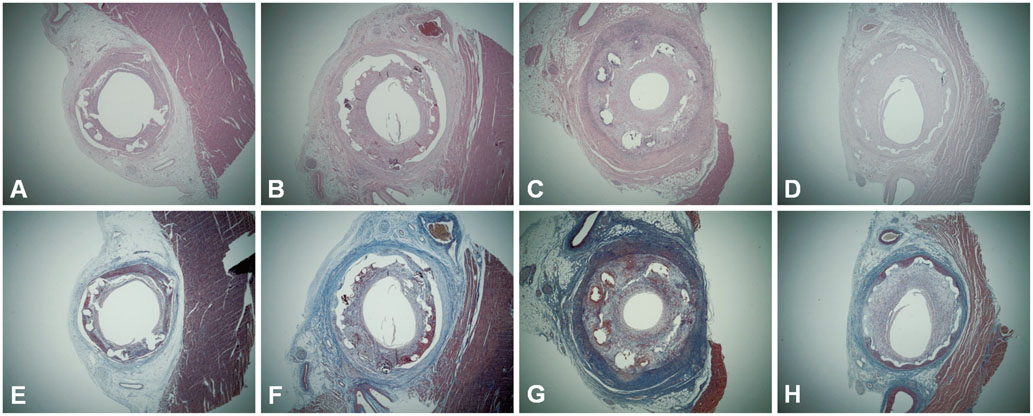
There were no significant differences between the pretreatment and no pretreatment groups in the internal elastic lamina area, lumen area, neointima area, stenotic area, injury score, fibrin score, and inflammation score. In both groups, the fibrin score was higher in pigs with DES than in BMS, particularly in ZES and EES. The inflammatory score was not different between DES and BMS.
CONCLUSION
In a porcine model of coronary restenosis, pretreatment with ezetimibe/simvastatin before DES implantation failed to improve arterial healing and endothelialization compared to treatment after stenting.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Safety and Efficacy of Biodegradable Polymer-biolimus-eluting Stents (BP-BES) Compared with Durable Polymer-everolimus-eluting Stents (DP-EES) in Patients Undergoing Complex Percutaneous Coronary Intervention
Pil Sang Song, Kyu Tae Park, Min Jeong Kim, Ki-Hyun Jeon, Jin-Sik Park, Rak Kyeong Choi, Young Bin Song, Seung-Hyuk Choi, Jin-Ho Choi, Sang Hoon Lee, Hyeon-Cheol Gwon, Jin-Ok Jeong, Eul Soon Im, Sang Wook Kim, Woo Jung Chun, Ju Hyeon Oh, Joo-Yong Hahn
Korean Circ J. 2019;49(1):69-80. doi: 10.4070/kcj.2018.0097.
Reference
-
1. Davidson MH, McGarry T, Bettis R, et al. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol. 2002; 40:2125–2134.2. Ballantyne CM, Houri J, Notarbartolo A, et al. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation. 2003; 107:2409–2415.3. Patti G, Pasceri V, Colonna G, et al. Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: results of the ARMYDA-ACS randomized trial. J Am Coll Cardiol. 2007; 49:1272–1278.4. Schwartz RS, Huber KC, Murphy JG, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol. 1992; 19:267–274.5. Kolodgie FD, John M, Khurana C, et al. Sustained reduction of in-stent neointimal growth with the use of a novel systemic nanoparticle paclitaxel. Circulation. 2002; 106:1195–1198.6. Finn AV, Kolodgie FD, Harnek J, et al. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation. 2005; 112:270–278.7. Drachman DE, Edelman ER, Seifert P, et al. Neointimal thickening after stent delivery of paclitaxel: change in composition and arrest of growth over six months. J Am Coll Cardiol. 2000; 36:2325–2332.8. Ridker PM, Rifai N, Pfeffer MA, et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1998; 98:839–844.9. Bustos C, Hernández-Presa MA, Ortego M, et al. HMG-CoA reductase inhibition by atorvastatin reduces neointimal inflammation in a rabbit model of atherosclerosis. J Am Coll Cardiol. 1998; 32:2057–2064.10. Dupuis J, Tardif JC, Cernacek P, Théroux P. Cholesterol reduction rapidly improves endothelial function after acute coronary syndromes. The RECIFE (reduction of cholesterol in ischemia and function of the endothelium) trial. Circulation. 1999; 99:3227–3233.11. Lacoste L, Lam JY, Hung J, Letchacovski G, Solymoss CB, Waters D. Hyperlipidemia and coronary disease. Correction of the increased thrombogenic potential with cholesterol reduction. Circulation. 1995; 92:3172–3177.12. Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1999; 100:230–235.13. Ridker PM, Rifai N, Lowenthal SP. Rapid reduction in C-reactive protein with cerivastatin among 785 patients with primary hypercholesterolemia. Circulation. 2001; 103:1191–1193.14. Albert MA, Danielson E, Rifai N, Ridker PM. PRINCE Investigators. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001; 286:64–70.15. Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999; 340:115–126.16. van der Wal AC, Becker AE, van der Loos CM, Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994; 89:36–44.17. Hong YJ, Jeong MH, Lee SR, et al. Anti-inflammatory effect of abciximab-coated stent in a porcine coronary restenosis model. J Korean Med Sci. 2007; 22:802–809.18. van Heek M, Farley C, Compton DS, Hoos L, Davis HR. Ezetimibe selectively inhibits intestinal cholesterol absorption in rodents in the presence and absence of exocrine pancreatic function. Br J Pharmacol. 2001; 134:409–417.19. Kerzner B, Corbelli J, Sharp S, et al. Efficacy and safety of ezetimibe coadministered with lovastatin in primary hypercholesterolemia. Am J Cardiol. 2003; 91:418–424.20. Davis HR Jr, Compton DS, Hoos L, Tetzloff G. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2001; 21:2032–2038.21. Nakagami H, Osako MK, Takami Y, et al. Vascular protective effects of ezetimibe in ApoE-deficient mice. Atherosclerosis. 2009; 203:51–58.22. Dietrich T, Hucko T, Bourayou R, et al. High resolution magnetic resonance imaging in atherosclerotic mice treated with ezetimibe. Int J Cardiovasc Imaging. 2009; 25:827–836.23. Graf K, Dietrich T, Tachezy M, et al. Monitoring therapeutical intervention with ezetimibe using targeted near-infrared fluorescence imaging in experimental atherosclerosis. Mol Imaging. 2008; 7:68–76.24. Gómez-Garre D, Muñoz-Pacheco P, González-Rubio ML, Aragoncillo P, Granados R, Fernández-Cruz A. Ezetimibe reduces plaque inflammation in a rabbit model of atherosclerosis and inhibits monocyte migration in addition to its lipid-lowering effect. Br J Pharmacol. 2009; 156:1218–1227.25. Davis HR Jr, Lowe RS, Neff DR. Effects of ezetimibe on atherosclerosis in preclinical models. Atherosclerosis. 2011; 215:266–278.26. Briand F, Naik SU, Fuki I, et al. Both the peroxisome proliferator-activated receptor delta agonist, GW0742, and ezetimibe promote reverse cholesterol transport in mice by reducing intestinal reabsorption of HDL-derived cholesterol. Clin Transl Sci. 2009; 2:127–133.27. Maekawa T, Komori K, Morisaki K, Itoh T. Ezetimibe reduces intimal hyperplasia in rabbit jugular vein graft. J Vasc Surg. 2012; 56:1689–1697.28. Cho JS, Jeong MH, Sim DS, et al. Effects of combined therapy with ezetimibe plus simvastatin after drug-eluting stent implantation in a porcine coronary restenosis model. J Korean Med Sci. 2010; 25:716–722.29. Park KH, Jeong MH, Kim JM, et al. The impact of triple anti-platelet therapy for endothelialization and inflammatory response at overlapping bioabsorbable polymer coated drug-eluting stents in a porcine coronary model. Int J Cardiol. 2013; 168:1853–1858.30. Lim KS, Jeong MH, Bae IH, et al. Histopathological comparison among biolimus, zotarolimus and everolimus-eluting stents in porcine coronary restenosis model. Korean Circ J. 2013; 43:744–751.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Systemic drug therapy and restenosis after drug-eluting stent implantation
- Effects of Combined Therapy with Ezetimibe Plus Simvastatin After Drug-Eluting Stent Implantation in a Porcine Coronary Restenosis Model
- Drug-Eluting Stent Strut Fracture as a Cause of Restenosis
- Coronary Restenosis after Drug-Eluting Stent Implantation in Diabetic Patients
- A Case of Stent Strut Fracture of a Paclitaxel-Eluting Stent at the Time of Stent Implantation in a Complex Coronary Lesion