Korean Circ J.
2016 Jan;46(1):23-32. 10.4070/kcj.2016.46.1.23.
Novel Fabrication of MicroRNA Nanoparticle-Coated Coronary Stent for Prevention of Post-Angioplasty Restenosis
- Affiliations
-
- 1Department of Biomedical Sciences and BK21 PLUS Center for Creative Biomedical Scientists, Gwangju, Korea. pik96@chonnam.ac.kr
- 2Heart Research Center, Chonnam National University Hospital, Gwangju, Korea.
- 3The Graduate School of Nanoscience and Technology and Department of Chemistry, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Korea.
- 4Center for Self-assembly and Complexicity, Department of Chemistry, Pohang University of Science and Technology, Pohang, Korea.
- KMID: 2223766
- DOI: http://doi.org/10.4070/kcj.2016.46.1.23
Abstract
- BACKGROUND AND OBJECTIVES
MicroRNA 145 is known to be responsible for cellular proliferation, and its enhanced expression reportedly inhibits the retardation of vascular smooth muscle cell growth specifically. In this study, we developed a microRNA 145 nanoparticle immobilized, hyaluronic acid (HA)-coated stent.
MATERIALS AND METHODS
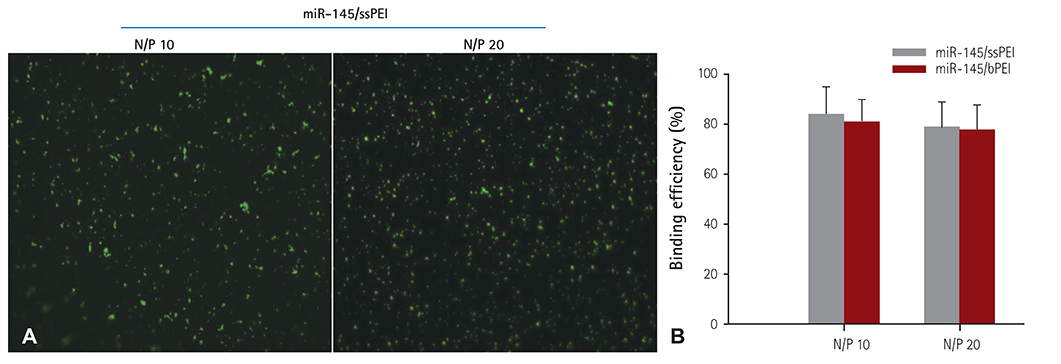
For the gene therapy, we used disulfide cross-linked low molecular polyethylenimine as the carrier. The microRNA 145 was labeled with YOYO-1 and the fluorescent microscopy images were obtained. The release of microRNA 145 from the stent was measured with an ultra violet spectrophotometer. The downstream targeting of the c-Myc protein and green fluorescent protein was determined by Western blotting. Finally, we deployed microRNA 145/ssPEI nanoparticles immobilized on HA-coated stents in the balloon-injured external iliac artery in a rabbit restenosis model.
RESULTS
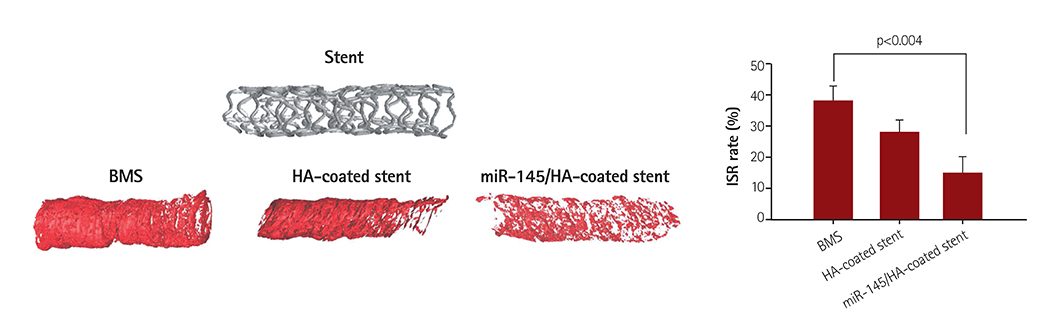
Cellular viability of the nanoparticle-immobilized surface tested using A10 vascular smooth muscle cells showed that MSN exhibited negligible cytotoxicity. In addition, microRNA 145 and downstream signaling proteins were identified by western blots with smooth muscle cell (SMC) lysates from the transfected A10 cell, as the molecular mechanism for decreased SMC proliferation that results in the inhibition of in-stent restenosis. MicroRNA 145 released from the stent suppressed the growth of the smooth muscle at the peri-stent implantation area, resulting in the prevention of restenosis at the post-implantation. We investigated the qualitative analyses of in-stent restenosis in the rabbit model using micro-computed tomography imaging and histological staining.
CONCLUSION
MicroRNA 145-eluting stent mitigated in-stent restenosis efficiently with no side effects and can be considered a successful substitute to the current drug-eluting stent.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Drug- and Gene-eluting Stents for Preventing Coronary Restenosis
Kamali Manickavasagam Lekshmi, Hui-Lian Che, Chong-Su Cho, In-Kyu Park
Chonnam Med J. 2017;53(1):14-27. doi: 10.4068/cmj.2017.53.1.14.
Reference
-
1. Thomas MK, Gupta YK. Drug-eluting stents: a pharmacoclinical perspective. Natl Med J India. 2006; 19:195–199.2. Zhu D, Jin X, Leng X, et al. Local gene delivery via endovascular stents coated with dodecylated chitosan-plasmid DNA nanoparticles. Int J Nanomedicine. 2010; 5:1095–1102.3. Mehran R, Dangas G, Abizaid AS, et al. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation. 1999; 100:1872–1878.4. Fischman DL, Leon MB, Baim DS, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994; 331:496–501.5. Babapulle MN, Eisenberg MJ. Coated stents for the prevention of restenosis: Part I. Circulation. 2002; 106:2734–2740.6. Nikol S. Possible uses of gene therapy in reducing coronary restenosis. Heart. 1997; 78:426–428.7. Gershlick AH. Drug eluting stents in 2005. Heart. 2005; 91(Suppl 3):iii24–iii31.8. Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002; 346:1773–1780.9. Lompré AM, Hadri L, Merlet E, et al. Efficient transduction of vascular smooth muscle cells with a translational AAV2.5 vector: A new perspective for in-stent restenosis gene therapy. Gene Ther. 2013; 20:901–912.10. Forbes SP, Alferiev IS, Chorny M, Adamo RF, Levy RJ, Fishbein I. Modulation of NO and ROS production by AdiNOS transduced vascular cells through supplementation with L-Arg and BH4: Implications for gene therapy of restenosis. Atherosclerosis. 2013; 230:23–32.11. Fishbein I, Chorny M, Adamo RF. Endovascular gene delivery from a stent platform: gene-eluting stents. Angiol Open Access. 2013; 1. pii:109.12. Brito LA, Chandrasekhar S, Little SR, Amiji MM. Non-viral eNOS gene delivery and transfection with stents for the treatment of restenosis. Biomed Eng Online. 2010; 9:56.13. Li JM, Newburger PE, Gounis MJ, Dargon P, Zhang X, Messina LM. Local arterial nanoparticle delivery of siRNA for NOX2 knockdown to prevent restenosis in an atherosclerotic rat model. Gene Ther. 2010; 17:1279–1287.14. Che HL, Bae IH, Lim KS, et al. Suppression of post-angioplasty restenosis with an Akt1 siRNA-embedded coronary stent in a rabbit model. Biomaterials. 2012; 33:8548–8556.15. Hwang do W, Son S, Jang J, et al. A brain-targeted rabies virus glycoprotein-disulfide linked PEI nanocarrier for delivery of neurogenic microRNA. Biomaterials. 2011; 32:4968–4975.16. Gouëffic Y, Guilluy C, Guérin P, Patra P, Pacaud P, Loirand G. Hyaluronan induces vascular smooth muscle cell migration through RHAMM-mediated PI3K-dependent Rac activation. Cardiovasc Res. 2006; 72:339–348.17. Muthiah M, Islam MA, Cho CS, Hwang JE, Chung IJ, Park IK. Substrate-mediated delivery of microRNA-145 through a polysorbitol-based osmotically active transporter suppresses smooth muscle cell proliferation: implications for restenosis treatment. J Biomed Nanotechnol. 2014; 10:571–579.18. Ye YC, Xie H, Zeng Y, Zhao X, Tian Z, Zhang S. Efficacy and safety of biodegradable polymer biolimus-eluting stents versus durable polymer drug-eluting stents: a meta-analysis. PLos One. 2013; 8:e78667.19. Al Ali J, Franck C, Filion KB, Eisenberg MJ. Efficacy and safety of drug eluting stents versus coronary artery bypass grafting: a meta-analysis of randomized controlled trials. Can J Cardiol. 2013; 29:S284.20. Yin RX, Yang DZ, Wu JZ. Nanoparticle drug- and gene-eluting stents for the prevention and treatment of coronary restenosis. Theranostics. 2014; 4:175–200.21. Hong YJ, Jeong MH, Ahn Y, Kang JC. The efficacy and safety of drug-eluting stents in patients with acute myocardial infarction: results from Korea Acute Myocardial Infarction (KAMIR). Int J Cardiol. 2013; 163:1–4.22. Paul A, Shao W, Shum-Tim D, Prakash S. The attenuation of restenosis following arterial gene transfer using carbon nanotube coated stent incorporating TAT/DNAAng1+VEGF nanoparticles. Biomaterials. 2012; 33:7655–7664.23. Lemkine GF, Demeneix BA. Polyethylenimines for in vivo gene delivery. Curr Opin Mol Ther. 2001; 3:178–182.24. Li D, Tang X, Pulli B, et al. Theranostic nanoparticles based on bioreducible polyethylenimine-coated iron oxide for reduction-responsive gene delivery and magnetic resonance imaging. Int J Nanomedicine. 2014; 9:3347–3361.25. Park S, Bhang SH, La WG, Seo J, Kim BS, Char K. Dual roles of hyaluronic acids in multilayer films capturing nanocarriers for drug-eluting coatings. Biomaterials. 2012; 33:5468–5477.26. Travis JA, Hughes MG, Wong JM, Wagner WD, Geary RL. Hyaluronan enhances contraction of collagen by smooth muscle cells and adventitial fibroblasts: role of CD44 and implications for constrictive remodeling. Circ Res. 2001; 88:77–83.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- No-Reflow Phenomenon During Treatment of Coronary In-Stent Restenosis With a Paclitaxel-Coated Balloon Catheter
- Meaning of serum antibody to Chlamydia pneumoniae in patients with restenosis after coronary balloon angioplasty or stent insertion
- Risk of Stent Stenosis after Implanting a First-Generation Drug-Eluting Stent and Drug Balloon Angioplasty
- Current Management of In-Stent Restenosis
- Percutaneous Coronary Intervention in Ischemic Heart Disease