J Rheum Dis.
2015 Aug;22(4):223-230. 10.4078/jrd.2015.22.4.223.
Incidence of Tuberculosis in Rheumatoid Arthritis Patients Using Anti-Tumor Necrosis Factor Agents following Latent Tuberculosis Infection Screening Strategies
- Affiliations
-
- 1Department of Rheumatology, Hanyang University Hospital for Rheumatic Diseases, Seoul, Korea. scbae@hanyang.ac.kr
- KMID: 2222852
- DOI: http://doi.org/10.4078/jrd.2015.22.4.223
Abstract
OBJECTIVE
To compare the incidence of tuberculosis (TB) in rheumatoid arthritis (RA) patients using tumor necrosis factor (TNF) inhibitors following two strategies for latent tuberculosis infection (LTBI) screening: Tuberculin skin test (TST) only vs. TST plus Qauntiferron-TB gold in tube (QFT-GIT).
METHODS
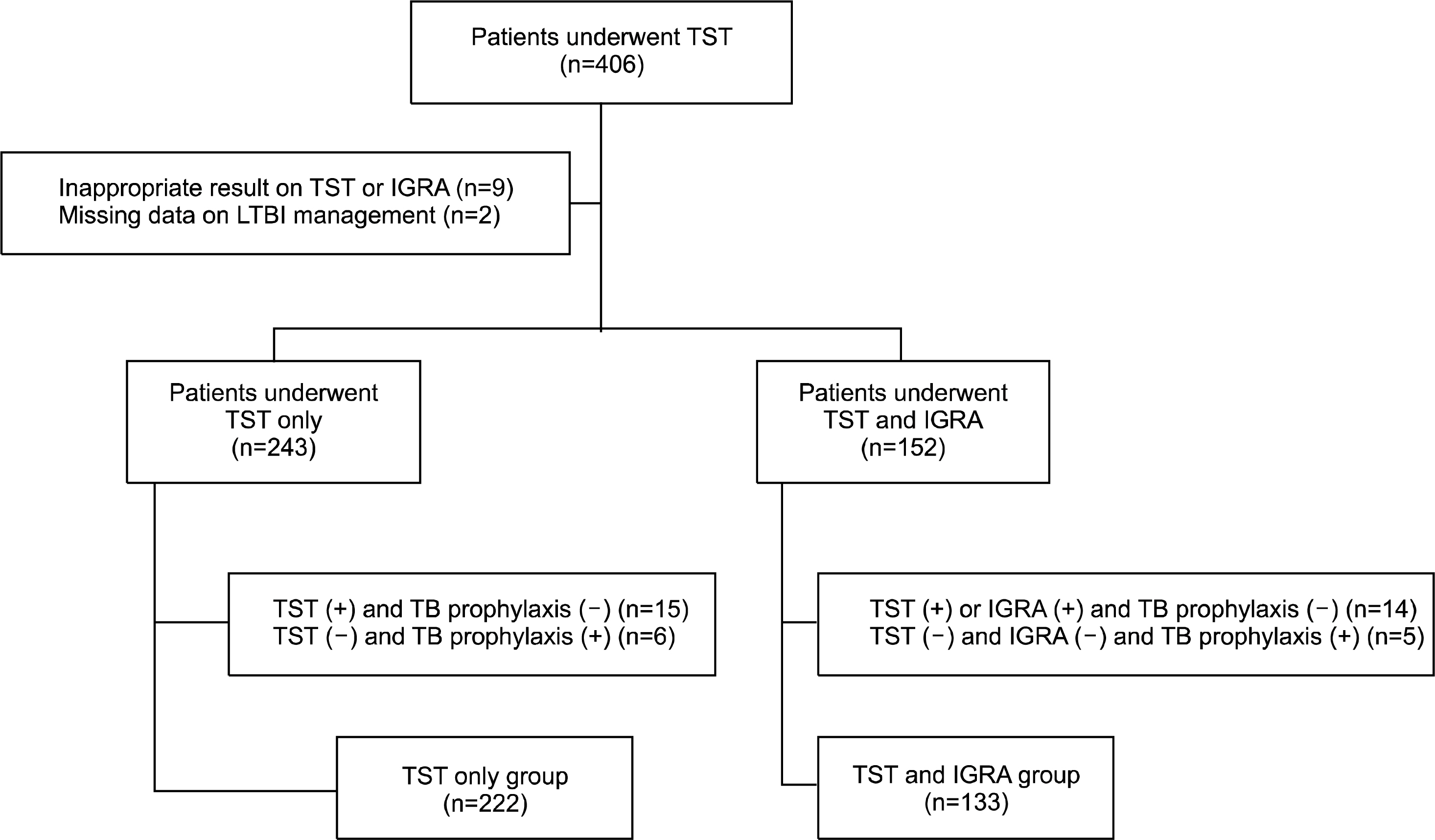
Data was extracted from a retrospective cohort of Korean RA patients who used biologic agents. Of the 406 RA patients who underwent TST before starting TNF inhibitor, we selected 355 patients who strictly followed LTBI screening and treatment strategies. Two hundred and twenty-two patients were classified as TST only group and the remaining 133 patients as TST plus QFT-GIT group. We calculated the standardized incidence ratio of TB in the entire sample and compared the TB incidence between groups.
RESULTS
Among the patients who received the TST only strategy (n=222, 538.6 person-year [PY]), two patients developed TB during anti-TNF therapy, while of those who followed the TST plus QFT-GIT strategy, none developed TB (n=133, 108.8 PY). The overall crude incidence of TB in RA patients using TNF inhibitors was 314 per 100,000 PY. Compared with the general population, the overall age standardized incidence of TB in TNF inhibitor users who followed management guideline for LTBI was 3.9.
CONCLUSION
Despite following screening and management guidelines for LTBI, TB incidence for RA patients during anti-TNF therapy is higher than in the general population. Combining QFT-GIT with TST as a screening for LTBI might be reduce the risk of TB.
MeSH Terms
Figure
Cited by 1 articles
-
Risk of Tuberculosis Development in Patients with Rheumatoid Arthritis Receiving Targeted Therapy: a Prospective Single Center Cohort Study
Yeo-Jin Song, Soo-Kyung Cho, Hyoungyoung Kim, Hye Won Kim, Eunwoo Nam, Sang-Cheol Bae, Dae Hyun Yoo, Yoon-Kyoung Sung
J Korean Med Sci. 2021;36(10):e70. doi: 10.3346/jkms.2021.36.e70.
Reference
-
1. Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor: Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999; 340:253–9.2. Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006; 54:26–37.
Article3. Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999; 354:1932–9.4. Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying anti-rheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008; 59:762–84.
Article5. Fallahi-Sichani M, El-Kebir M, Marino S, Kirschner DE, Linderman JJ. Multiscale computational modeling reveals a critical role for TNF-α receptor 1 dynamics in tuberculosis granuloma formation. J Immunol. 2011; 186:3472–83.
Article6. Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001; 345:1098–104.7. Gómez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD. BIOBADASER Group. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum. 2003; 48:2122–7.
Article8. Wolfe F, Michaud K, Anderson J, Urbansky K. Tuberculosis infection in patients with rheumatoid arthritis and the effect of infliximab therapy. Arthritis Rheum. 2004; 50:372–9.
Article9. Askling J, Fored CM, Brandt L, Baecklund E, Bertilsson L, Cöster L, et al. Risk and case characteristics of tuberculosis in rheumatoid arthritis associated with tumor necrosis factor antagonists in Sweden. Arthritis Rheum. 2005; 52:1986–92.
Article10. Dixon WG, Hyrich KL, Watson KD, Lunt M, Galloway J, Ustianowski A. B S R B R Control Centre Consortium, Symmons DP; BSR Biologics Register. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR). Ann Rheum Dis. 2010; 69:522–8.
Article11. Seong SS, Choi CB, Woo JH, Bae KW, Joung CL, Uhm WS, et al. Incidence of tuberculosis in Korean patients with rheumatoid arthritis (RA): effects of RA itself and of tumor necrosis factor blockers. J Rheumatol. 2007; 34:706–11.12. Mohan AK, Coté TR, Block JA, Manadan AM, Siegel JN, Braun MM. Tuberculosis following the use of etanercept, a tumor necrosis factor inhibitor. Clin Infect Dis. 2004; 39:295–9.
Article13. Keane J. Tumor necrosis factor blockers and reactivation of latent tuberculosis. Clin Infect Dis. 2004; 39:300–2.
Article14. Chen DY, Shen GH, Hsieh TY, Hsieh CW, Lan JL. Effectiveness of the combination of a whole-blood interfer-on-gamma assay and the tuberculin skin test in detecting latent tuberculosis infection in rheumatoid arthritis patients receiving adalimumab therapy. Arthritis Rheum. 2008; 59:800–6.
Article15. Iannone F, Cantini F, Lapadula G. Diagnosis of latent tuberculosis and prevention of reactivation in rheumatic patients receiving biologic therapy: international recommendations. J Rheumatol Suppl. 2014; 91:41–6.
Article16. Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012; 64:625–39.
Article17. Korean Guidelines for Tuberculosis. 1st ed.Seoul: Committee for the Development of Korean Guidelines for Tuberculosis Korea Centers for Disease Control and Prevention;2011. p. 174.18. Korean Guidelines for Tuberculosis. 2nd ed.Seoul: Committee for the Revision of Korean Guidelines for Tuberculosis Korea Centers for Disease Control and Prevention;2014. p. 194.19. Kim JH, Cho SK, Han M, Choi CB, Kim TH, Jun JB, et al. Factors influencing discrepancies between the Quanti-FERON-TB gold in tube test and the tuberculin skin test in Korean patients with rheumatic diseases. Semin Arthritis Rheum. 2013; 42:424–32.
Article20. Brassard P, Kezouh A, Suissa S. Antirheumatic drugs and the risk of tuberculosis. Clin Infect Dis. 2006; 43:717–22.
Article21. Carmona L, Hernández-García C, Vadillo C, Pato E, Balsa A, González-Alvaro I, et al. EMECAR Study Group. Increased risk of tuberculosis in patients with rheumatoid arthritis. J Rheumatol. 2003; 30:1436–9.22. Shim TS, Koh WJ, Yim JJ, Lew WJ. Treatment of latent tuberculosis infection in Korea. Tuberc Respir Dis. 2008; 65:79–90.
Article23. Favalli EG, Caporali R, Sinigaglia L, Pipitone N, Miniati I, Montecucco C, et al. Italian Society for Rheumatology. Recommendations for the use of biologic therapy in rheumatoid arthritis: update from the Italian Society for Rheumatology II. Safety. Clin Exp Rheumatol. 2011; 29(3 Suppl 66):S15–27.24. Bombardier C, Hazlewood GS, Akhavan P, Schieir O, Dooley A, Haraoui B, et al. Canadian Rheumatology Association. Canadian Rheumatology Association recommendations for the pharmacological management of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs: part II safety. J Rheumatol. 2012; 39:1583–602.
Article25. Beglinger C, Dudler J, Mottet C, Nicod L, Seibold F, Villiger PM, et al. Screening for tuberculosis infection before the initiation of an anti-TNF-alpha therapy. Swiss Med Wkly. 2007; 137:620–2.26. Solovic I, Sester M, Gomez-Reino JJ, Rieder HL, Ehlers S, Milburn HJ, et al. The risk of tuberculosis related to tumour necrosis factor antagonist therapies: a TBNET consensus statement. Eur Respir J. 2010; 36:1185–206.
Article27. Updated recommendations for the use of biological agents for the treatment of rheumatic diseases [Internet]. Sydney: Australian Rheumatology Association, reveised. 2011; [cited 2014 Feb 24]. Available from:. http://rheumatology.org.au/downloads/FINAL-BiologicalRecommendations-060111.xml.28. Ponce de León D, Acevedo-Vásquez E, Sánchez-Torres A, Cucho M, Alfaro J, Perich R, et al. Attenuated response to purified protein derivative in patients with rheumatoid arthritis: study in a population with a high prevalence of tuberculosis. Ann Rheum Dis. 2005; 64:1360–1.29. Mow WS, Abreu-Martin MT, Papadakis KA, Pitchon HE, Targan SR, Vasiliauskas EA. High incidence of anergy in inflammatory bowel disease patients limits the usefulness of PPD screening before infliximab therapy. Clin Gastroenterol Hepatol. 2004; 2:309–13.
Article30. Carmona L, Gómez-Reino JJ, Rodríguez-Valverde V, Montero D, Pascual-Gómez E, Mola EM, et al. BIOBADASER Group. Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum. 2005; 52:1766–72.
Article31. Bélard E, Semb S, Ruhwald M, Werlinrud AM, Soborg B, Jensen FK, et al. Prednisolone treatment affects the performance of the QuantiFERON gold in-tube test and the tuberculin skin test in patients with autoimmune disorders screened for latent tuberculosis infection. Inflamm Bowel Dis. 2011; 17:2340–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Guidelines for Prevention of Tuberculosis in Patients with Rheumatoid Arthritis Treated with TNF-alpha Blockers
- A Case of Multiple Tuberculosis Associated with Infliximab Therapy in Crohn's Disease
- Diagnosis and Treatment of Latent Tuberculosis Infection due to Initiation of Anti-TNF Therapy
- Diagnosis and Treatment of Latent Tuberculosis Infection in Patients with Inflammatory Bowel Diseases due to Initiation of Anti-Tumor Necrosis Factor Therapy
- Rectal tuberculosis after infliximab therapy despite negative screening for latent tuberculosis in a patient with ulcerative colitis