Allergy Asthma Respir Dis.
2015 Nov;3(6):439-445. 10.4168/aard.2015.3.6.439.
Fractional exhaled nitric oxide in Korean children with allergic rhinitis
- Affiliations
-
- 1Department of Pediatrics, Inha University School of Medicine, Incheon, Korea. kimjhmd@inha.ac.kr
- 2Environmental Health Center for Allergic Rhinitis, Inha University Hospital, Incheon, Korea.
- KMID: 2218633
- DOI: http://doi.org/10.4168/aard.2015.3.6.439
Abstract
- PURPOSE
Fractional exhaled nitric oxide (FeNO) is useful for the diagnosis of allergic rhinitis (AR) as well as bronchial asthma (BA). However, FeNO may differ according to race, age, and other determinants. There have been few studies about FeNO in Korean children with AR. The aims of this study were to evaluate the value of FeNO in AR and to compare FeNO, and determinants of FeNO levels between AR, BA, and combined AR and BA.
METHODS
This study included 647 children aged 5 to 17. The children were classified into 5 groups after performing the skin test, FeNO measurement, the pulmonary function test, and the methacholine challenge test: those with nonallergic rhinitis (NAR), those with AR, those with BA, and those with combined AR and BA, and healthy controls,.
RESULTS
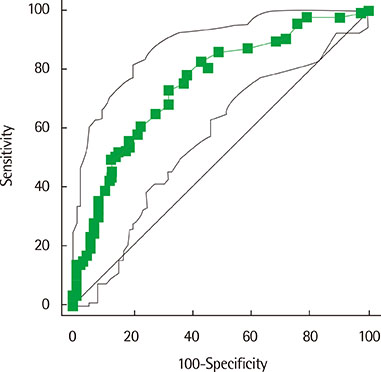
The values of FEV1 (forced expiratory volume in one second) %predicted were 94.4%+/-12.6%, 93.8%+/-20.7%, 90.0%+/-17.4% in AR, BA, and combined AR and BA, respectively. The values of FeNO in AR (32.3+/-25.0 ppb), BA (31.1+/-20.5 ppb), and combined AR and BA (34.5+/-30.4 ppb) were significantly higher compared to those of NAR (16.8+/-13.5 ppb) and controls (15.9+/-12.5 ppb). There was no significant difference in FeNO among AR, BA, and combined AR and BA. FeNO was significantly higher in patients with > or =4 positive results (36.6+/-29.2 ppb) than in those with <4 positive skin test results (27.6+/-20.7 ppb). When the receiver operating characteristic curve analysis for prediction of AR showed 0.756 of area under the curve, the cutoff level of FeNO was 16 ppb.
CONCLUSION
In this study, children with AR had increased levels of FeNO. It is suggested that AR may have eosinophilic bronchial inflammation without BHR or clinical asthma.
MeSH Terms
Figure
Cited by 2 articles
-
Clinical application of fractional exhaled nitric oxide in pediatric allergic rhinitis
Doo Hee Han
Allergy Asthma Respir Dis. 2015;3(6):385-386. doi: 10.4168/aard.2015.3.6.385.Clinical characteristics of allergic rhinitis and nonallergic rhinitis in Korean children
Na Hae Won, Sang Hyun Park, So Hyun Ahn, Chae Bong Kim, Jung Hyun Kwon, Won Hee Seo, Dae Jin Song, Young Yoo
Allergy Asthma Respir Dis. 2020;8(1):20-29. doi: 10.4168/aard.2020.8.1.20.
Reference
-
1. Jee HM, Kim KW, Kim CS, Sohn MH, Shin DC, Kim KE. Prevalence of asthma, rhinitis and eczema in Korean Children using the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaires. Pediatr Allergy Respir Dis. 2009; 19:165–172.2. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008; 63:Suppl 86. 8–160.3. Passalacqua G, Ciprandi G, Canonica GW. The nose-lung interaction in allergic rhinitis and asthma: united airways disease. Curr Opin Allergy Clin Immunol. 2001; 1:7–13.
Article4. van den Nieuwenhof L, Schermer T, Bosch Y, Bousquet J, Heijdra Y, Bor H, et al. Is physician-diagnosed allergic rhinitis a risk factor for the development of asthma? Allergy. 2010; 65:1049–1055.
Article5. Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol. 2003; 111:1171–1183.
Article6. Stewart L, Katial RK. Exhaled nitric oxide. Immunol Allergy Clin North Am. 2012; 32:347–362.
Article7. Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011; 184:602–615.
Article8. Hanson JR, De Lurgio SA, Williams DD, Dinakar C. Office-based exhaled nitric oxide measurement in children 4 years of age and older. Ann Allergy Asthma Immunol. 2013; 111:358–363.
Article9. Linhares D, Jacinto T, Pereira AM, Fonseca JA. Effects of atopy and rhinitis on exhaled nitric oxide values: a systematic review. Clin Transl Allergy. 2011; 1:8.
Article10. Sonnappa S, Bastardo CM, Stafler P, Bush A, Aurora P, Stocks J. Ethnic differences in fraction of exhaled nitric oxide and lung function in healthy young children. Chest. 2011; 140:1325–1331.
Article11. Kovesi T, Kulka R, Dales R. Exhaled nitric oxide concentration is affected by age, height, and race in healthy 9- to 12-year-old children. Chest. 2008; 133:169–175.
Article12. Yao TC, Lee WI, Ou LS, Chen LC, Yeh KW, Huang JL, et al. Reference values of exhaled nitric oxide in healthy Asian children aged 5 to 18 years. Eur Respir J. 2012; 39:378–384.
Article13. La Grutta S, Ferrante G, Malizia V, Cibella F, Viegi G. Environmental effects on fractional exhaled nitric oxide in allergic children. J Allergy (Cairo). 2012; 2012:916926.
Article14. van Weel C, Bateman ED, Bousquet J, Reid J, Grouse L, Schermer T, et al. Asthma management pocket reference 2008. Allergy. 2008; 63:997–1004.
Article15. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005; 26:319–338.
Article16. Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000; 161:309–329.17. Son BK, Lim DH. Allergic skin test. Korean J Pediatr. 2007; 50:409–415.
Article18. American Thoracic Society. European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005; 171:912–930.19. Cobos Barroso N, Perez-Yarza EG, Sardon Prado O, Reverte Bover C, Gartner S, Korta Murua J. Exhaled nitric oxide in children: a noninvasive marker of airway inflammation. Arch Bronconeumol. 2008; 44:41–51.
Article20. Leynaert B, Neukirch C, Kony S, Guenegou A, Bousquet J, Aubier M, et al. Association between asthma and rhinitis according to atopic sensitization in a population-based study. J Allergy Clin Immunol. 2004; 113:86–93.
Article21. Ciprandi G, Tosca MA, Capasso M. Exhaled nitric oxide in children with allergic rhinitis and/or asthma: a relationship with bronchial hyperreactivity. J Asthma. 2010; 47:1142–1147.
Article22. Kalpaklioglu AF, Kalkan IK. Comparison of orally exhaled nitric oxide in allergic versus nonallergic rhinitis. Am J Rhinol Allergy. 2012; 26:e50–e54.23. Chawes BL, Bonnelykke K, Kreiner-Moller E, Bisgaard H. Children with allergic and nonallergic rhinitis have a similar risk of asthma. J Allergy Clin Immunol. 2010; 126:567–573. e1–e8.
Article24. Makinen T, Lehtimaki L, Kinnunen H, Nieminen R, Kankaanranta H, Moilanen E. Bronchial diffusing capacity of nitric oxide is increased in patients with allergic rhinitis. Int Arch Allergy Immunol. 2009; 148:154–160.
Article25. Kim YH, Park HB, Kim MJ, Kim HS, Lee HS, Han YK, et al. Fractional exhaled nitric oxide and impulse oscillometry in children with allergic rhinitis. Allergy Asthma Immunol Res. 2014; 6:27–32.
Article26. Cirillo I, Ricciardolo FL, Medusei G, Signori A, Ciprandi G. Exhaled nitric oxide may predict bronchial hyperreactivity in patients with allergic rhinitis. Int Arch Allergy Immunol. 2013; 160:322–328.
Article27. Aronsson D, Tufvesson E, Bjermer L. Allergic rhinitis with or without concomitant asthma: difference in perception of dyspnoea and levels of fractional exhaled nitric oxide. Clin Exp Allergy. 2005; 35:1457–1461.
Article28. Linn WS, Rappaport EB, Berhane KT, Bastain TM, Avol EL, Gilliland FD. Exhaled nitric oxide in a population-based study of southern California schoolchildren. Respir Res. 2009; 10:28.
Article29. Cho HJ, Jung YH, Yang SI, Lee E, Kim HY, Seo JH, et al. Reference values and determinants of fractional concentration of exhaled nitric oxide in healthy children. Allergy Asthma Immunol Res. 2014; 6:169–174.
Article30. van Amsterdam JG, Janssen NA, de Meer G, Fischer PH, Nierkens S, van Loveren H, et al. The relationship between exhaled nitric oxide and allergic sensitization in a random sample of school children. Clin Exp Allergy. 2003; 33:187–191.
Article31. Clark AT, Ewan PW. Interpretation of tests for nut allergy in one thousand patients, in relation to allergy or tolerance. Clin Exp Allergy. 2003; 33:1041–1045.
Article32. Rouhos A, Kainu A, Karjalainen J, Lindqvist A, Piirila P, Sarna S, et al. Atopic sensitization to common allergens without symptoms or signs of airway disorders does not increase exhaled nitric oxide. Clin Respir J. 2008; 2:141–148.
Article33. Ko HS, Choi SH, Rha YH. Role of fractional exhaled nitric oxide in predicting development of allergic rhinits in children with bronchial asthma. Pediatr Allergy Respir Dis. 2012; 22:180–187.
Article34. Malinovschi A, Alving K, Kalm-Stephens P, Janson C, Nordvall L. Increased exhaled nitric oxide predicts new-onset rhinitis and persistent rhinitis in adolescents without allergic symptoms. Clin Exp Allergy. 2012; 42:433–440.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical application of fractional exhaled nitric oxide in pediatric allergic rhinitis
- Changes in levels of fractional exhaled and nasal nitric oxide after treatment in allergic rhinitis
- Nasal and Exhaled Nitric Oxide in Allergic Rhinitis
- Role of Fractional Exhaled Nitric Oxide in Predicting Development of Allergic Rhinits in Children with Bronchial Asthma
- Exhaled NO: Determinants and Clinical Application in Children With Allergic Airway Disease