Allergy Asthma Respir Dis.
2015 Nov;3(6):387-395. 10.4168/aard.2015.3.6.387.
Recent advances in the classification and management of hypereosinophilia
- Affiliations
-
- 1Department of Internal Medicine, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea.
- 2Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea. allergy21@hotmail.com
- KMID: 2218626
- DOI: http://doi.org/10.4168/aard.2015.3.6.387
Abstract
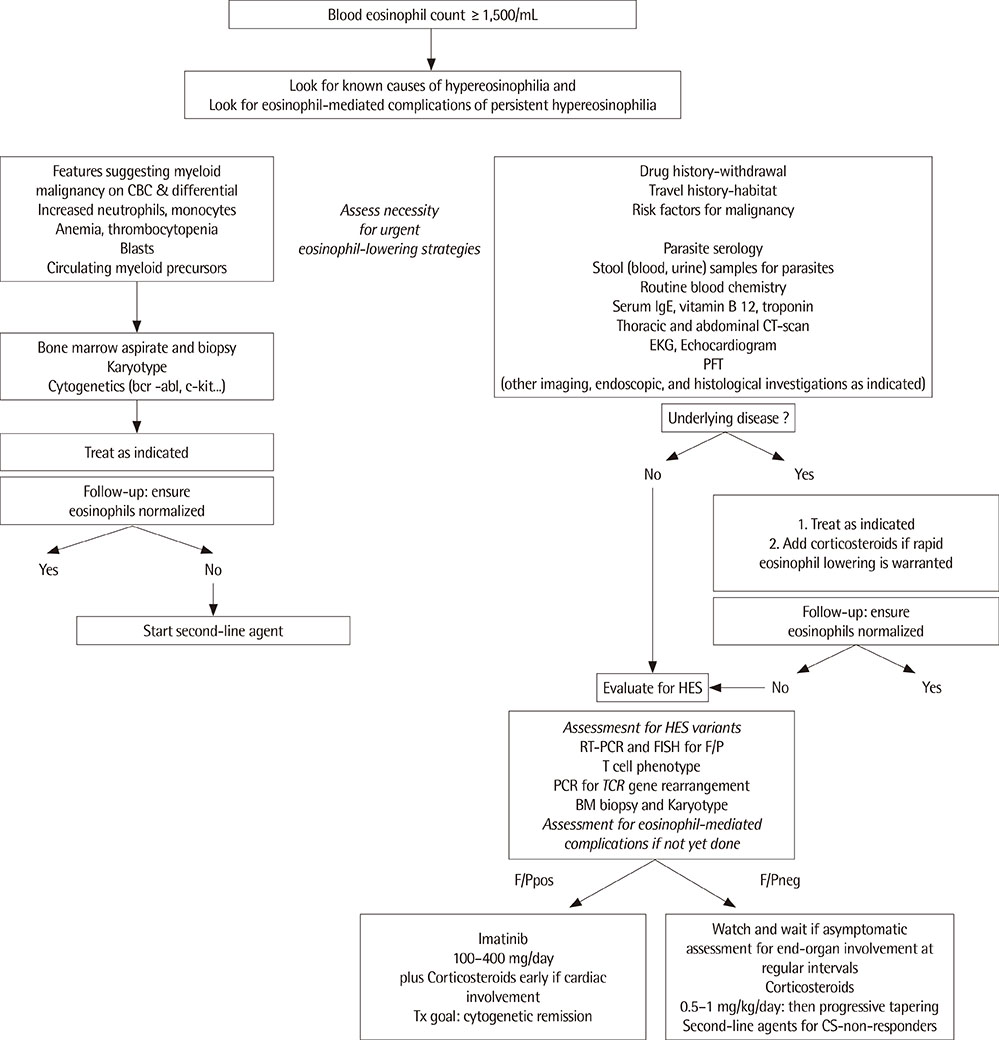
- Numerous disorders and etiologies may underlie increased eosinophil counts. Hypereosinophilia (HE) is defined as a peripheral blood eosinophil count greater than 1,500/mm3 and may be potentially harmful because of tissue damage. Hypereosinophilic syndrome (HES) also represents a heterogeneous disorder characterized by persistent HE with the evidence of organ dysfunction, clinical symptoms, or both caused by eosinophilia. The refining criteria and subclassification of HE and HES are currently being revised on cellular and molecular based diagnostic methods. Initial approaches focus on evaluating various underlying causes, including helminthic infections, adverse drug reactions, allergic diseases, and neoplastic diseases. When secondary causes of HE are excluded, the workup should proceed to the evaluation of primary/clonal bone marrow disease, including fip 1-like 1-platelet driven growth factor receptor alpha (FIP1L1-PDGFRA) mutation. Concurrently, if the patient has symptoms and signs, organ damage or dysfunction must be evaluated. Although, corticosteroids are the mainstay of therapy in confirmed HES, imatinib is considered a definitive treatment for FIP1L1-PDGFRA, platelet driven growth factor receptor beta rearranged HE and HES. In this article, we discuss recent advances in the classification of and practical approaches to HE and HES. In addition, we introduce several promising therapies for HE and HES.
MeSH Terms
Figure
Cited by 1 articles
-
Anaplastic large cell lymphoma with marked peripheral eosinophilia misdiagnosed as Kimura disease
Yoonji Shin, Jee Youn Oh, Young Seok Lee, Kyung Hoon Min, Sung Yong Lee, Jae Jeong Shim, Kyung Ho Kang, Gyu Young Hur
Allergy Asthma Respir Dis. 2018;6(2):131-134. doi: 10.4168/aard.2018.6.2.131.
Reference
-
1. Kita H. Eosinophils: multifaceted biological properties and roles in health and disease. Immunol Rev. 2011; 242:161–177.
Article2. Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008; 38:709–750.
Article3. Valent P, Gleich GJ, Reiter A, Roufosse F, Weller PF, Hellmann A, et al. Pathogenesis and classification of eosinophil disorders: a review of recent developments in the field. Expert Rev Hematol. 2012; 5:157–176.
Article4. Brigden M, Graydon C. Eosinophilia detected by automated blood cell counting in ambulatory North American outpatients. Incidence and clinical significance. Arch Pathol Lab Med. 1997; 121:963–967.5. Rothenberg ME. Eosinophilia. N Engl J Med. 1998; 338:1592–1600.
Article6. Chusid MJ, Dale DC, West BC, Wolff SM. The hypereosinophilic syndrome: analysis of fourteen cases with review of the literature. Medicine (Baltimore). 1975; 54:1–27.7. Valent P, Klion AD, Horny HP, Roufosse F, Gotlib J, Weller PF, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012; 130:607–612.e9.
Article8. Park CS, Kim TB, Cho YS, Moon HB. Diagnosis and treatment of eosinophilia: a guideline for Korean patients. Korean J Asthma Allergy Clin Immunol. 2006; 26:186–197.9. Choi DC. Eosinophilia. Korean J Asthma Allergy Clin Immunol. 2011; 31:237–245.10. Lee JY, Yang MH, Hwang JH, Kang M, Paeng JW, Yune S, et al. The prevalence of toxocariasis and diagnostic value of serologic tests in asymptomatic Korean adults. Allergy Asthma Immunol Res. 2015; 7:467–475.
Article11. Kwon NH, Oh MJ, Lee SP, Lee BJ, Choi DC. The prevalence and diagnostic value of toxocariasis in unknown eosinophilia. Ann Hematol. 2006; 85:233–238.
Article12. Kwon NH, Oh MJ, Lee SP, Lee BJ, Choi DC. The clinical impact of toxocariasis in patients with unknown eosinophilia. Korean J Asthma Allergy Clin Immunol. 2005; 25:299–304.13. Curtis C, Ogbogu PU. Evaluation and differential diagnosis of persistent marked eosinophilia. Immunol Allergy Clin North Am. 2015; 35:387–402.
Article14. Tefferi A, Patnaik MM, Pardanani A. Eosinophilia: secondary, clonal and idiopathic. Br J Haematol. 2006; 133:468–492.
Article15. Todenhofer T, Wirths S, von Weyhern CH, Heckl S, Horger M, Hennenlotter J, et al. Severe paraneoplastic hypereosinophilia in metastatic renal cell carcinoma. BMC Urol. 2012; 12:7.
Article16. Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003; 348:1201–1214.
Article17. Gotlib J. World Health Organization-defined eosinophilic disorders: 2014 update on diagnosis, risk stratification, and management. Am J Hematol. 2014; 89:325–337.
Article18. Roufosse F, Weller PF. Practical approach to the patient with hypereosinophilia. J Allergy Clin Immunol. 2010; 126:39–44.
Article19. Ogbogu PU, Bochner BS, Butterfield JH, Gleich GJ, Huss-Marp J, Kahn JE, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009; 124:1319–1325.e3.
Article20. Flaum MA, Schooley RT, Fauci AS, Gralnick HR. A clinicopathologic correlation of the idiopathic hypereosinophilic syndrome. I. Hematologic manifestations. Blood. 1981; 58:1012–1020.
Article21. Akuthota P, Weller PF. Spectrum of eosinophilic end-organ manifestations. Immunol Allergy Clin North Am. 2015; 35:403–411.
Article22. Wilkins HJ, Crane MM, Copeland K, Williams WV. Hypereosinophilic syndrome: an update. Am J Hematol. 2005; 80:148–157.
Article23. Hsieh FH. Hypereosinophilic syndrome. Ann Allergy Asthma Immunol. 2014; 112:484–488.
Article24. Roufosse F, Schandene L, Sibille C, Willard-Gallo K, Kennes B, Efira A, et al. Clonal Th2 lymphocytes in patients with the idiopathic hypereosinophilic syndrome. Br J Haematol. 2000; 109:540–548.
Article25. Lefebvre C, Bletry O, Degoulet P, Guillevin L, Bentata-Pessayre M, Le Thi Huong Du, et al. Prognostic factors of hypereosinophilic syndrome. Study of 40 cases. Ann Med Interne (Paris). 1989; 140:253–257.26. Podjasek JC, Butterfield JH. Mortality in hypereosinophilic syndrome: 19 years of experience at Mayo Clinic with a review of the literature. Leuk Res. 2013; 37:392–395.
Article27. Fauci AS, Harley JB, Roberts WC, Ferrans VJ, Gralnick HR, Bjornson BH. NIH conference. The idiopathic hypereosinophilic syndrome. Clinical, pathophysiologic, and therapeutic considerations. Ann Intern Med. 1982; 97:78–92.28. Lee SP. Hightlights and diagnostic dilemma of toxocariasis. Korean J Med. 2013; 84:200–202.
Article29. Klion AD, Noel P, Akin C, Law MA, Gilliland DG, Cools J, et al. Elevated serum tryptase levels identify a subset of patients with a myeloproliferative variant of idiopathic hypereosinophilic syndrome associated with tissue fibrosis, poor prognosis, and imatinib responsiveness. Blood. 2003; 101:4660–4666.
Article30. Noguchi H, Kephart GM, Colby TV, Gleich GJ. Tissue eosinophilia and eosinophil degranulation in syndromes associated with fibrosis. Am J Pathol. 1992; 140:521–528.31. Kato M, Kephart GM, Talley NJ, Wagner JM, Sarr MG, Bonno M, et al. Eosinophil infiltration and degranulation in normal human tissue. Anat Rec. 1998; 252:418–425.
Article32. Tefferi A, Gotlib J, Pardanani A. Hypereosinophilic syndrome and clonal eosinophilia: point-of-care diagnostic algorithm and treatment update. Mayo Clin Proc. 2010; 85:158–164.
Article33. Malbrain ML, Van den Bergh H, Zachee P. Further evidence for the clonal nature of the idiopathic hypereosinophilic syndrome: complete haematological and cytogenetic remission induced by interferon-alpha in a case with a unique chromosomal abnormality. Br J Haematol. 1996; 92:176–183.
Article34. Helbig G, Hus M, Halasz M, Dudzinski M, Wieclawek A, Stachowicz M, et al. Imatinib mesylate may induce long-term clinical response in FIP1L1-PDGFRα-negative hypereosinophilic syndrome. Med Oncol. 2012; 29:1073–1076.
Article35. Pitini V, Arrigo C, Azzarello D, La Gattuta G, Amata C, Righi M, et al. Serum concentration of cardiac Troponin T in patients with hypereosinophilic syndrome treated with imatinib is predictive of adverse outcomes. Blood. 2003; 102:3456–3457.
Article36. Plotz SG, Simon HU, Darsow U, Simon D, Vassina E, Yousefi S, et al. Use of an anti-interleukin-5 antibody in the hypereosinophilic syndrome with eosinophilic dermatitis. N Engl J Med. 2003; 349:2334–2339.
Article37. Rothenberg ME, Klion AD, Roufosse FE, Kahn JE, Weller PF, Simon HU, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008; 358:1215–1228.
Article38. Roufosse FE, Kahn JE, Gleich GJ, Schwartz LB, Singh AD, Rosenwasser LJ, et al. Long-term safety of mepolizumab for the treatment of hypereosinophilic syndromes. J Allergy Clin Immunol. 2013; 131:461–467.e1-5.
Article39. Kolbeck R, Kozhich A, Koike M, Peng L, Andersson CK, Damschroder MM, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010; 125:1344–1353.e2.40. Wilson TM, Maric I, Shukla J, Brown M, Santos C, Simakova O, et al. IL-5 receptor α levels in patients with marked eosinophilia or mastocytosis. J Allergy Clin Immunol. 2011; 128:1086–1092.e1-3.
Article41. Legrand F, Klion AD. Biologic therapies targeting eosinophils: current status and future prospects. J Allergy Clin Immunol Pract. 2015; 3:167–174.
Article42. Strati P, Cortes J, Faderl S, Kantarjian H, Verstovsek S. Long-term follow-up of patients with hypereosinophilic syndrome treated with Alemtuzumab, an anti-CD52 antibody. Clin Lymphoma Myeloma Leuk. 2013; 13:287–291.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent advances in diagnosis and therapy in systemic mastocytosis
- Recent changes in the concept of treatment of insomnia
- Recent advances in ischemic stroke management
- Recent Advances in Immunotherapy of Lung Cancer
- A Rare Cause of Peripheral Vascular Thrombosis: Hypereosinophilia Caused by Toxocara canis Infection