J Korean Ophthalmol Soc.
2016 Mar;57(3):499-506. 10.3341/jkos.2016.57.3.499.
Expression of Nestin on Endothelial Cells and Pericytes During Retinal Vascular Development in Mouse
- Affiliations
-
- 1Department of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea. steph25@snu.ac.kr
- 2Fight against Angiogenesis-Related Blindness Laboratory, Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea.
- 3Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Medicine, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2213253
- DOI: http://doi.org/10.3341/jkos.2016.57.3.499
Abstract
- PURPOSE
Nestin, a marker of neural stem cells, is expressed in Müller cells during retinal development. However, the role of nestin in retinal vascular development is not well established. Thus, we investigated the expression of nestin in developmental mouse retina and identified which retinal cells are related to the expression of nestin during the retinal vascular development.
METHODS
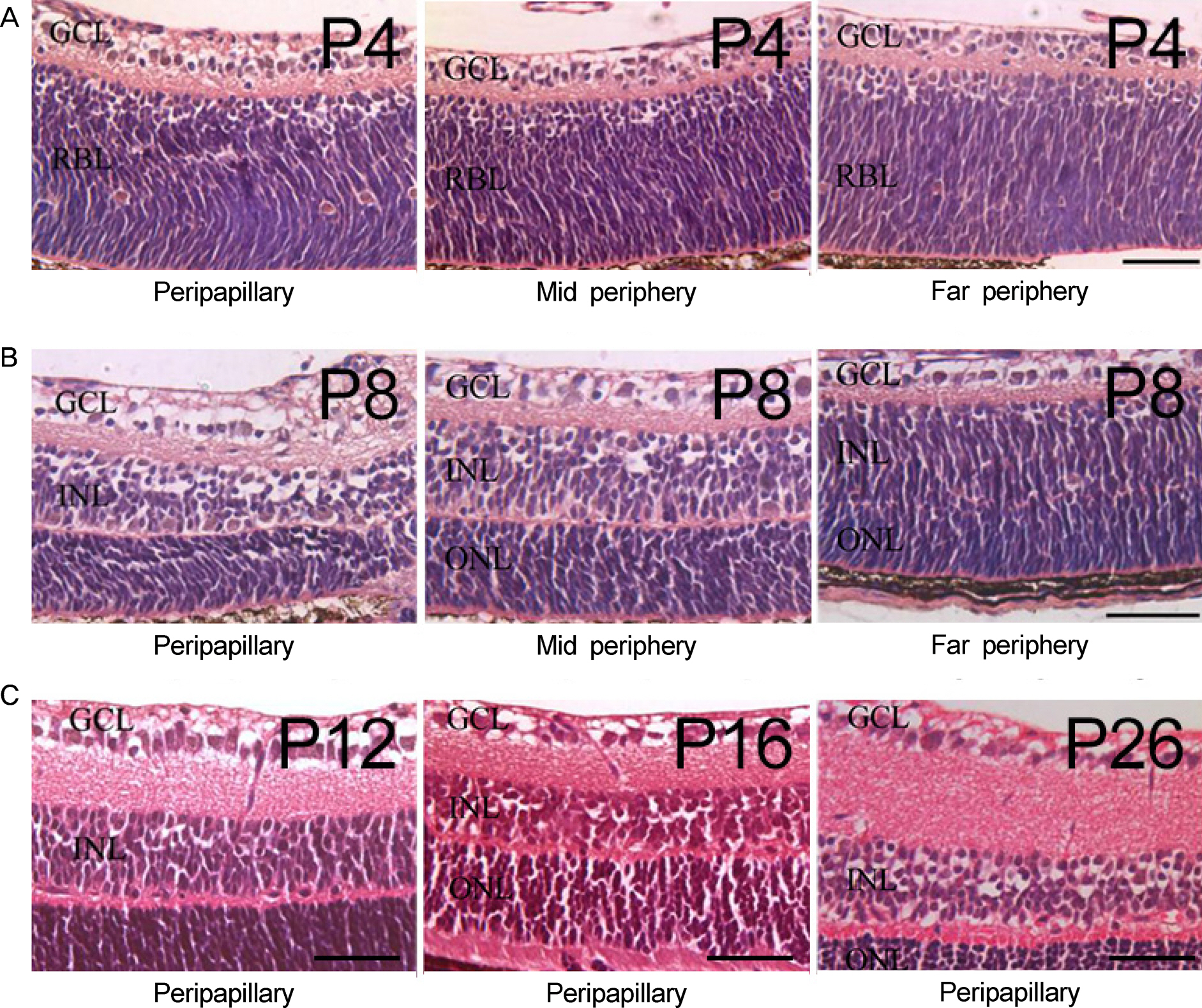
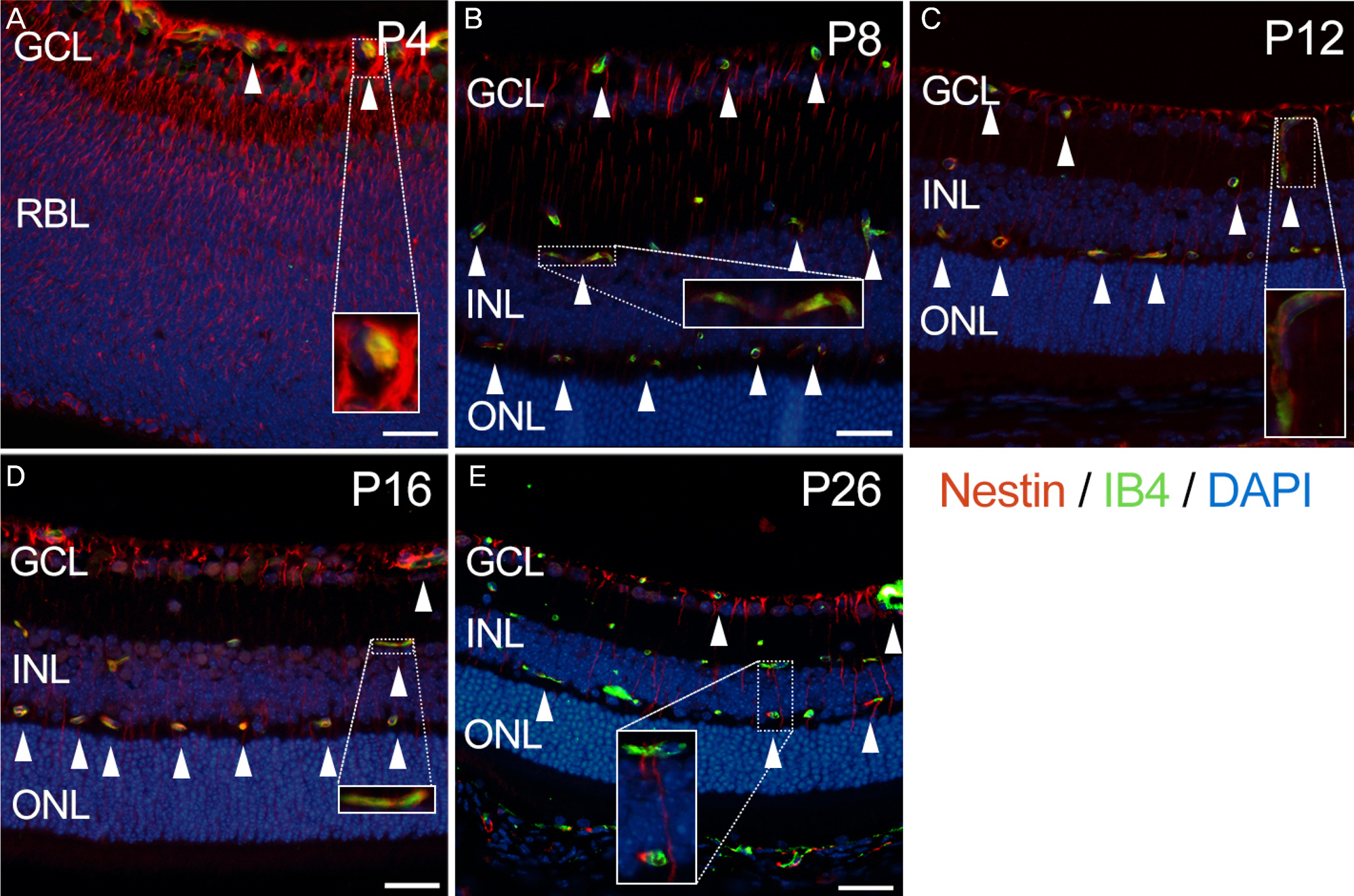
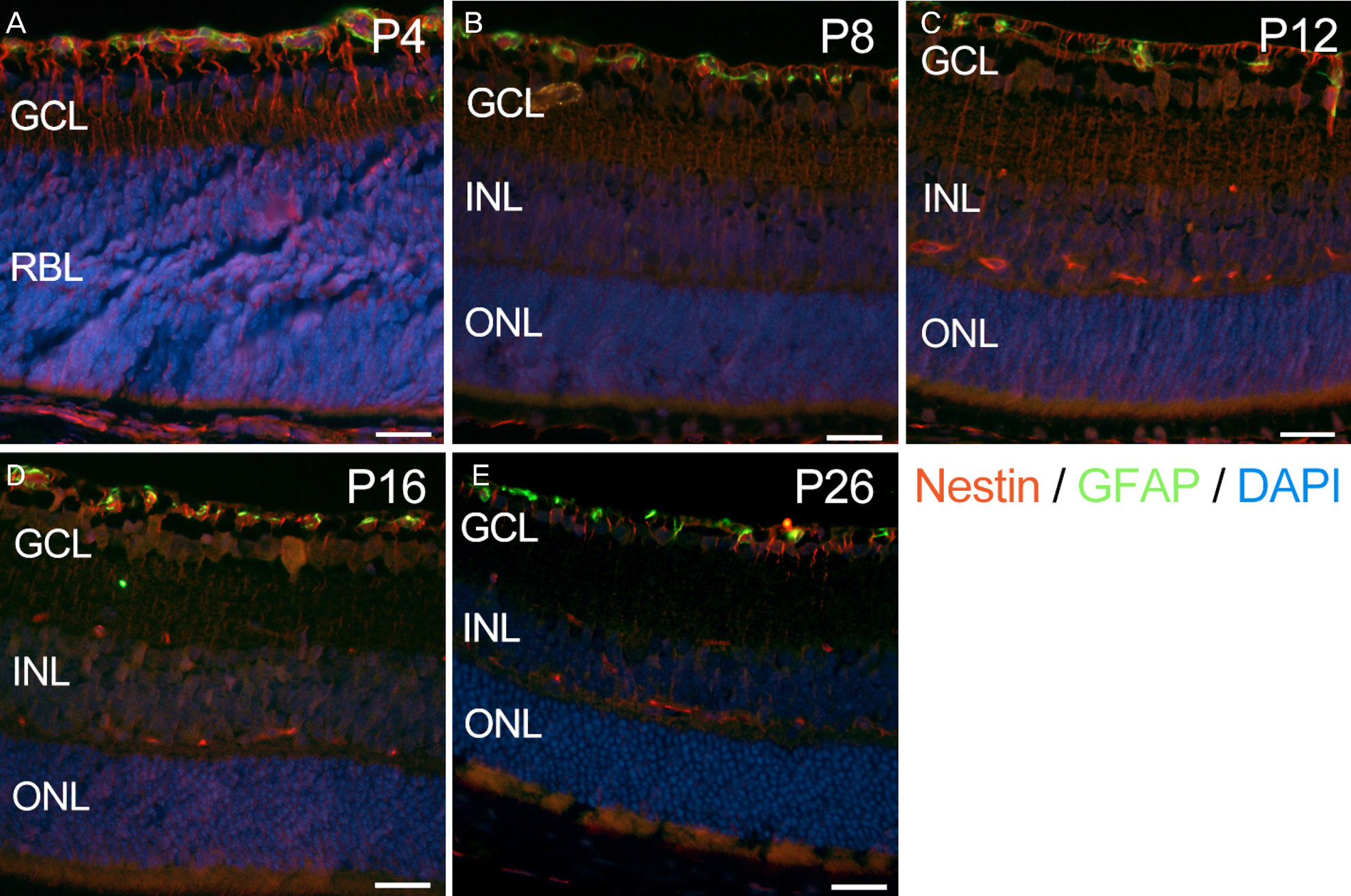
Eyes were enucleated from C57BL/6 mice on postnatal day (P) 4, P8, P12, P16 and P26. Immunofluorescence was used to evaluate nestin expression in relation to endothelial cells (isolectin B4), pericytes (neural/glial antigen 2) and astrocytes (glial fibrillary acidic protein).
RESULTS
Nestin was strongly expressed from the ganglion cell layer to retinoblast layer at P4. At P8, P12 and P16, the expression of nestin was observed from the upper border of the ganglion cell layer, and vertically penetrating to outer nuclear layer. At P26, the expression of nestin was decreased and confined to the ganglion cell layer and inner nuclear layer. Interestingly, there was strong vascular shape expression of nestin at all stages. The superficial, deep and intermediate vascular plexus was completely merged with nestin expression at P4, P8, P12 and P16. In addition, the nestin expression merged with pericytes but not with astrocytes.
CONCLUSIONS
Nestin was expressed in endothelial cells and pericytes during retinal vascular development in the retina. These results suggest that nestin could play an important role in developmental angiogenesis via interplay with endothelial cells and pericytes.
MeSH Terms
Figure
Reference
-
References
1. Guillemin K, Krasnow MA. The hypoxic response: huffing and HIFing. Cell. 1997; 89:9–12.
Article2. Muñoz B, West SK, Rubin GS, et al. Causes of blindness and visual impairment in a population of older Americans: The Salisbury Eye Evaluation Study. Arch Ophthalmol. 2000; 118:819–25.3. Yoon KC, Mun GH, Kim SD, et al. Prevalence of eye diseases in South Korea: data from the Korea National Health and Nutrition Examination Survey 2008-2009. Korean J Ophthalmol. 2011; 25:421–33.
Article4. Jee D, Lee WK, Kang S. Prevalence and risk factors for diabetic retinopathy: the Korea National Health and Nutrition Examination Survey 2008-2011. Invest Ophthalmol Vis Sci. 2013; 54:6827–33.
Article5. Park SJ, Lee JH, Woo SJ, et al. Age-related macular degeneration: prevalence and risk factors from Korean National Health and Nutrition Examination Survey, 2008 through 2011. Ophthalmology. 2014; 121:1756–65.6. Chan-Ling T, Gock B, Stone J. The effect of oxygen on vasoformative cell division. Evidence that ‘physiological hypoxia’ is the stimulus for normal retinal vasculogenesis. Invest Ophthalmol Vis Sci. 1995; 36:1201–14.7. Stone J, Itin A, Alon T, et al. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995; 15(7 Pt 1):4738–47.
Article8. Dorrell MI, Aguilar E, Friedlander M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest Ophthalmol Vis Sci. 2002; 43:3500–10.9. Kim JH, Kim JH, Yu YS, et al. Recruitment of pericytes and astrocytes is closely related to the formation of tight junction in developing retinal vessels. J Neurosci Res. 2009; 87:653–9.
Article10. Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990; 60:585–95.
Article11. Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985; 5:3310–28.
Article12. Terling C, Rass A, Mitsiadis TA, et al. Expression of the intermediate filament nestin during rodent tooth development. Int J Dev Biol. 1995; 39:947–56.13. Kachinsky AM, Dominov JA, Miller JB. Myogenesis and the intermediate filament protein, nestin. Dev Biol. 1994; 165:216–28.
Article14. Sejersen T, Lendahl U. Transient expression of the intermediate filament nestin during skeletal muscle development. J Cell Sci. 1993; 106(Pt 4):1291–300.
Article15. Suzuki S, Namiki J, Shibata S, et al. The neural stem/progenitor cell marker nestin is expressed in proliferative endothelial cells, but not in mature vasculature. J Histochem Cytochem. 2010; 58:721–30.
Article16. Yamahatsu K, Matsuda Y, Ishiwata T, et al. Nestin as a novel therapeutic target for pancreatic cancer via tumor angiogenesis. Int J Oncol. 2012; 40:1345–57.
Article17. Dorrell MI, Friedlander M. Mechanisms of endothelial cell guidance and vascular patterning in the developing mouse retina. Prog Retin Eye Res. 2006; 25:277–95.
Article18. Lee JH, Park HS, Shin JM, et al. Nestin expressing progenitor cells during establishment of the neural retina and its vasculature. Anat Cell Biol. 2012; 45:38–46.
Article19. Wohl SG, Schmeer CW, Kretz A, et al. Optic nerve lesion increases cell proliferation and nestin expression in the adult mouse eye in vivo. Exp Neurol. 2009; 219:175–86.
Article20. Steinert PM, Chou YH, Prahlad V, et al. A high molecular weight intermediate filament-associated protein in BHK-21 cells is nestin, a type VI intermediate filament protein. Limited co-assembly in vitro to form heteropolymers with type III vimentin and type IV alpha-internexin. J Biol Chem. 1999; 274:9881–90.21. Marvin MJ, Dahlstrand J, Lendahl U, McKay RD. A rod end deletion in the intermediate filament protein nestin alters its subcellular localization in neuroepithelial cells of transgenic mice. J Cell Sci. 1998; 111(Pt 14):1951–61.
Article22. Liang ZW, Wang Z, Chen H, et al. Nestin-mediated cytoskeletal remodeling in endothelial cells: novel mechanistic insight into VEGF-induced cell migration in angiogenesis. Am J Physiol Cell Physiol. 2015; 308:C349–58.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nestin expressing progenitor cells during establishment of the neural retina and its vasculature
- Cellularities of the Retinal Capillaries
- Expression of Nestin in the Rat Kidney with Ischemia-Reperfusion Injury
- Relationship between Pericytes and Endothelial Cells in Retinal Neovascularization: A Histological and Immunofluorescent Study of Retinal Angiogenesis
- Nestin Expression in the Adult Mouse Retina with Pharmaceutically Induced Retinal Degeneration