J Korean Ophthalmol Soc.
2009 Dec;50(12):1881-1886. 10.3341/jkos.2009.50.12.1881.
Methazolamide-induced Stevens-Johnson Syndrome
- Affiliations
-
- 1Department of Ophthalmology, Dankook University College of Medicine, Cheonan, Korea. Happyeye21@medimail.co.kr
- KMID: 2213003
- DOI: http://doi.org/10.3341/jkos.2009.50.12.1881
Abstract
- PURPOSE
To report three consecutive cases of methazolamide-induced Stevens-Johnson syndrome.
CASE SUMMARY
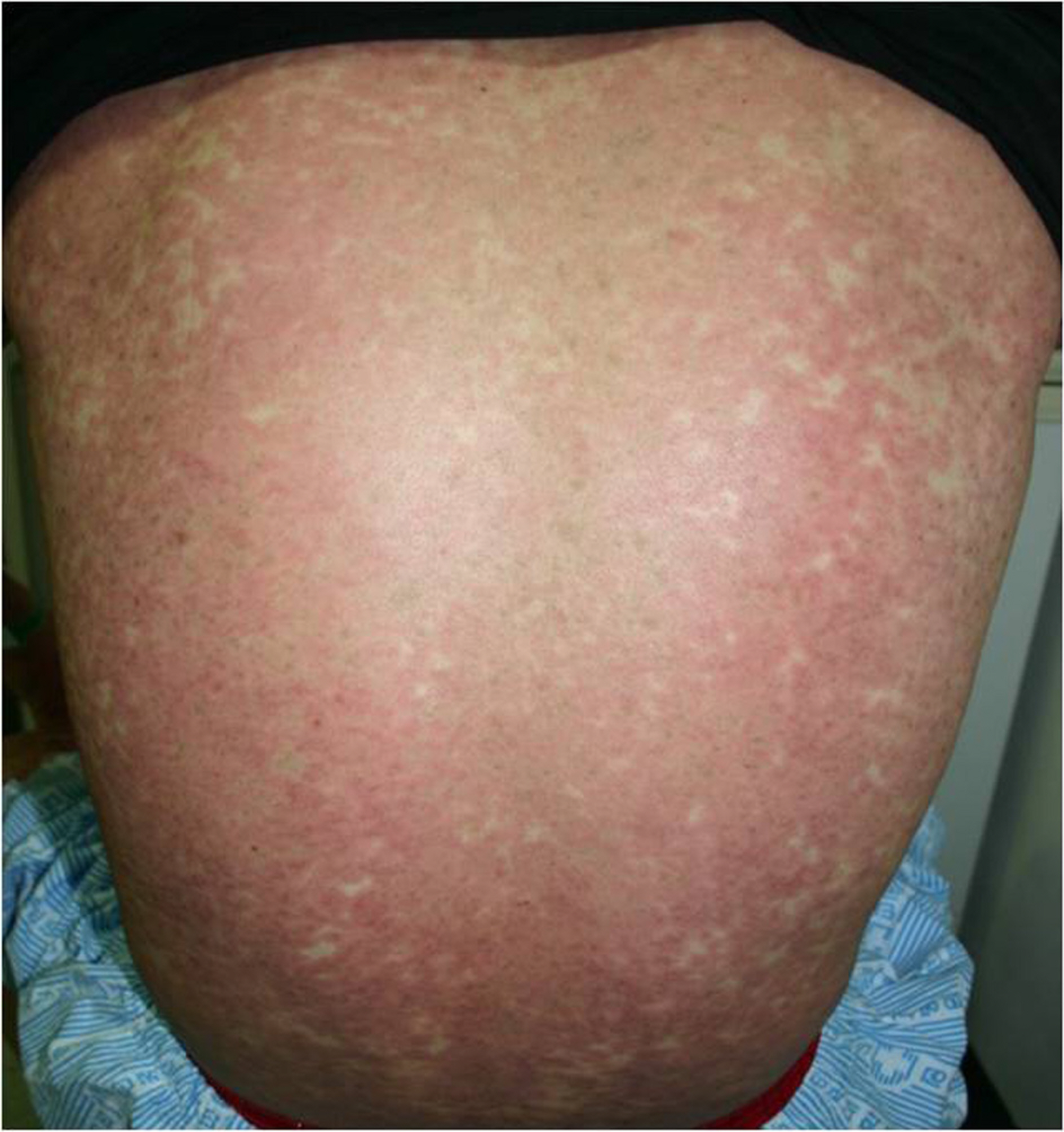
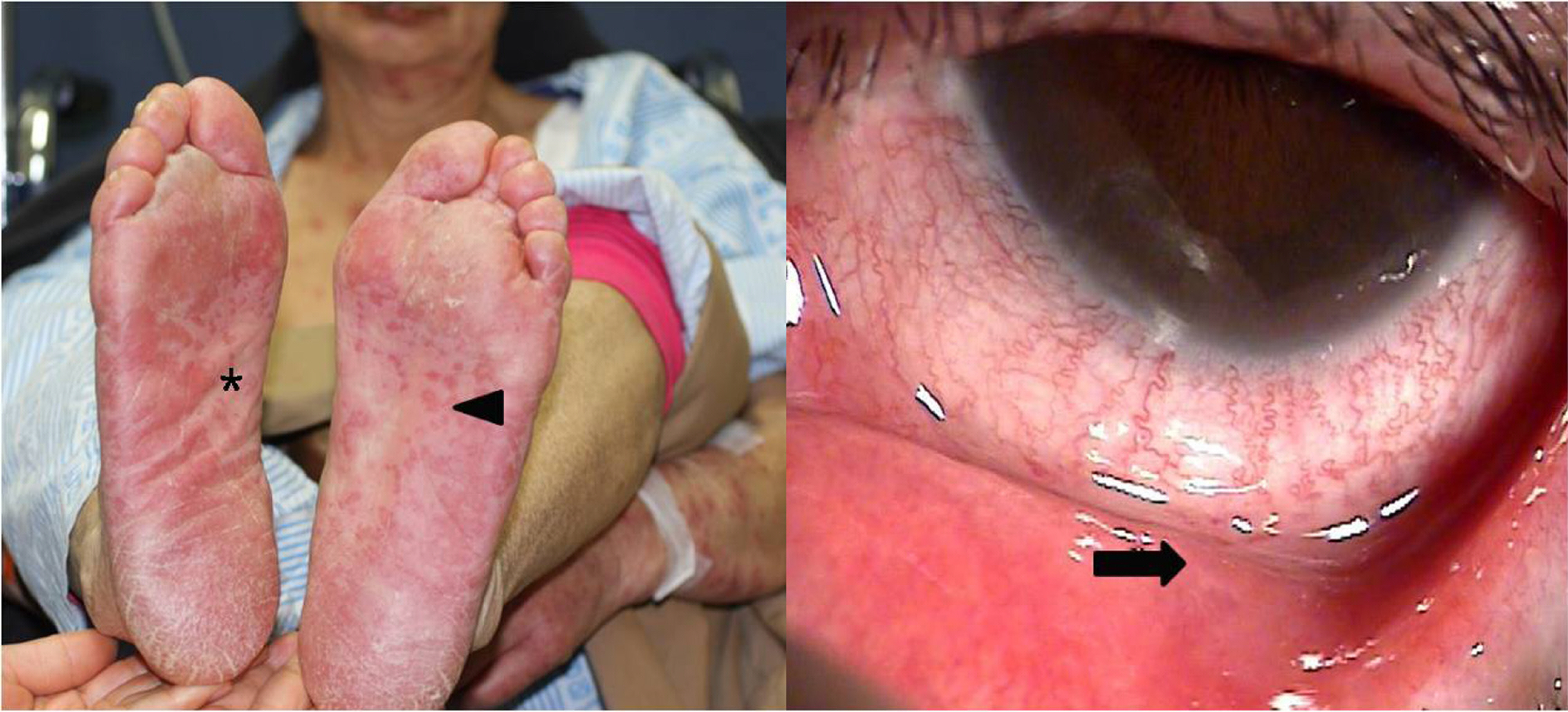
We describe three patients who were all prescribed methazolamide for treatment of ophthalmologic conditions. A 29-year-old man and a 47- year-old woman were prescribed methazolamide (100 mg/day) for the treatment of central serous chorioretinopathy (CSCR). A 66-year-old woman was prescribed methazolamide (100 mg/day) for acute glaucoma of the left eye for approximately two weeks. After taking the methazolamide, three patients were showed the pururitic maculopapular rashes on the whole body and the vesicular eruptions of the oral mucosa and conjunctiva. On the basis of medication histories, characteristic skin lesions and mucosal involvement, we diagnosed all three patients with methazolamide-induced Stevens-Johnson syndrome. All three patients were hospitalized and treated with intravenous steroids and antihistamines. Two of the three cases showed conjunctival pseudomembranes. In two cases, the skin lesions worsened during the first week of treatment, and then resolved without complications over the next two to three weeks. The condition of the 47-year-old female patient deteriorated rapidly to toxic epidermal necrolysis due to sensitivity to sulfa antibiotics. HLA- A24, B59 and Cw1 were detected in all three cases.
CONCLUSIONS
In 2008, domestic production of acetazolamide was halted in Korea. Because of this, methazolamide is expected to be prescribed by ophthalmologists more commonly than in previous years. Complete medical histories should be taken before prescribing methazolamide to patients. HLA typing should be conducted whenever possible to screen patients before prescription of methazolamide.
Keyword
MeSH Terms
-
Acetazolamide
Adult
Aged
Anti-Bacterial Agents
Central Serous Chorioretinopathy
Conjunctiva
Epidermal Necrolysis, Toxic
Exanthema
Eye
Female
Glaucoma
Histamine Antagonists
Histocompatibility Testing
HLA-B Antigens
Humans
Korea
Methazolamide
Middle Aged
Mouth Mucosa
Prescriptions
Skin
Steroids
Stevens-Johnson Syndrome
Acetazolamide
Anti-Bacterial Agents
HLA-B Antigens
Histamine Antagonists
Methazolamide
Steroids
Figure
Cited by 1 articles
-
Methazolamide-induced toxic epidermal necrolysis confirmed by lymphocyte activation test
Kyu-Hyung Han, Ku-Hyun Hong, Doh Hyung Kim, Youn Seup Kim, Jae-Suk Park, Seung-Heon Kim, Young-Koo Jee
Allergy Asthma Respir Dis. 2016;4(4):301-304. doi: 10.4168/aard.2016.4.4.301.
Reference
-
References
1. Stevens AM, Johnson FC. A new eruptive fever associated with stomatitis and ophthalmia. Am J Dis Child. 1922; 24:526–33.
Article2. Brilakis HS, Palmon FE, Wbster GF, Hollan EJ. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. Krachmer JH, Mannis MJ, Holland EJ, editors. Cornea. 2nd ed.Philadelphia: ELSEVIER MOSBY;2005. 1:chap. 58.3. Abe R. Toxic epidermal necrolysis and Stevens-Johnson syndrome: Soluble Fas ligand involvement in the pathomechanisms of these diseases. J Dermatol Sci. 2008; 52:151–9.
Article4. Borchers AT, Lee JL, Naguwa SM, et al. Stevens–Johnson syndrome and toxic epidermal necrolysis. Autoimmun Rev. 2008; 7:598–605.
Article5. Chan HL, Stern RS, Arndt KA, et al. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. Arch Dermatol. 1990; 126:43–7.
Article6. Chan LS, Soong HK, Foster CS, et al. Ocular cicatricial pemphigoid occurring as a sequela of Stevens-Johnson syndrome. JAMA. 1991; 266:1543–6.
Article7. Schöpf E, Stühmer A, Rzany B, et al. Toxic epidermal necrolysis and Stevens-Johnson syndrome. An epidemiologic study from West Germany. Arch Dermatol. 1991; 127:839–42.8. Bianchine JR, Macaraeg PV Jr, Lasagna L, et al. Drugs as etiologic factors in the Stevens-Johnson syndrome. Am J Med. 1968; 44:390–405.
Article9. Tonnesen MG, Soter NA. Erythema multiforme. J Am Acad Dermatol. 1979; 1:357–64.
Article10. Raviglione MC, Pablos MA, Battan R. Clinical features and management of severe dermatological reactions to drugs. Drug Saf. 1990; 5:39–64.
Article11. Dunagin WG, Millikan LE. Drug eruption. Med Clin North Am. 1980; 64:983–1003.12. Genvert GI, Cohen EJ, Donnenfeld ED, Blecher MH. Erythema multiforme after use of topical sulfacetamide. Am J Ophthalmol. 1985; 99:465–8.
Article13. Ostler HB, Conant MA, Groundwater J. Lyell's disease, the Stevens-Johnson syndromes, and exfoliative dermatitis. Trans Am Acad Ophthalmol Otolaryongo. 1970; 74:1254–65.14. Shelley WB. Herpes simplex virus as a cause of erythema multiforme. JAMA. 1967; 201:153–6.
Article15. Maren TH, Haywood JR, Chapman SK, Zimmerman TJ. The pharmacology of methazolamide in relation to the treatment of glaucoma. Invest Ophthalmol Vis Sci. 1977; 16:730–42.16. Moon JI, Seo JH, Park CK. Association of HLA type with Stevens-Johnson syndrome induced by methazolamide treatment. J Korean Ophthalmol Soc. 2000; 41:2241–6.17. Flach AJ, Smith RE, Fraunfelder FT. Stevens-Johnson syndrome associated with methazolamide treatment reported in two Japanese-American women. Ophthalmology. 1995; 102:1677–80.
Article18. Shirato S, Kagaya F, Suzuki Y, Joukou S. Stevens-Johnson syndrome induced by methazolamide treatment. Arch Ophthalmol. 1997; 115:550–3.
Article19. Park YJ, Moon JI, Park CK. Three cases of Stevens-Johnson syndrome associated with methazolamide treatment. J Korean ophthalmol Soc. 1999; 40:613–8.20. Ueta M, Tokunaga K, Sotozono C, et al. HLA class I and II gene polymorphisms in Stevens-Johnson syndrome with ocular complications in Japanese. Mol Vis. 2008; 14:550–5.21. Roujeau JC, Huynh TN, Bracq C, et al. Genetic susceptibility to toxic epidermal necrolysis. Arch Dermatol. 1987; 123:1171–3.
Article22. Whang DH, Yang YS, Hong HK. Allele and haplotype frequencies of human leukocyte antigen-A, -B, and -DR loci in Koreans: DNA typing of 1,500 cord blood units. Korean J Lab Med. 2008; 28:465–74.
Article23. Middleton D, Menchaca L, Rood H, Komerofsky R. New allele frequency database. Tissue Antigens. 2003; 61:403–7.24. Kourlas H, Morey S. Sulfonamide allergy and possible cross-reactivity. Journal of Pharmacy Practice. 20:399–402.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Five Cases of Stevens-Johnson Syndrome May Be Associated with Methazolamide Treatment
- Four cases of Stevens-Johnson Syndrome associated with Methazolamide Treatment
- Two Cases of HLA-B59(+) Stevens-Johnson Syndrome (SJS)-Toxic Epidermal Necrolysis (TEN) Associated with Methazolamide Treatment
- Three Cases of Stevens-Johnson Syndrome Associated with Methazolamide Treatment
- Association of HLA Type with Stevens-Johnson Syndrome Induced by Methazolamide Treatment