J Korean Ophthalmol Soc.
2009 Oct;50(10):1489-1494. 10.3341/jkos.2009.50.10.1489.
Cinical Effect of Restasis(R) Eye Drops in Mild Dry Eye Syndrome
- Affiliations
-
- 1Department of Ophthalmology, School of Medicine, Pusan National University, Busan, Korea. jongsool@pusan.ac.kr
- 2Shinsaegae Eye Clinic, Ulsan, Korea.
- KMID: 2212765
- DOI: http://doi.org/10.3341/jkos.2009.50.10.1489
Abstract
- PURPOSE
To evaluate changes in tear secretion and symptoms in patients with mild dry eye syndrome after using Restasis(R).
METHODS
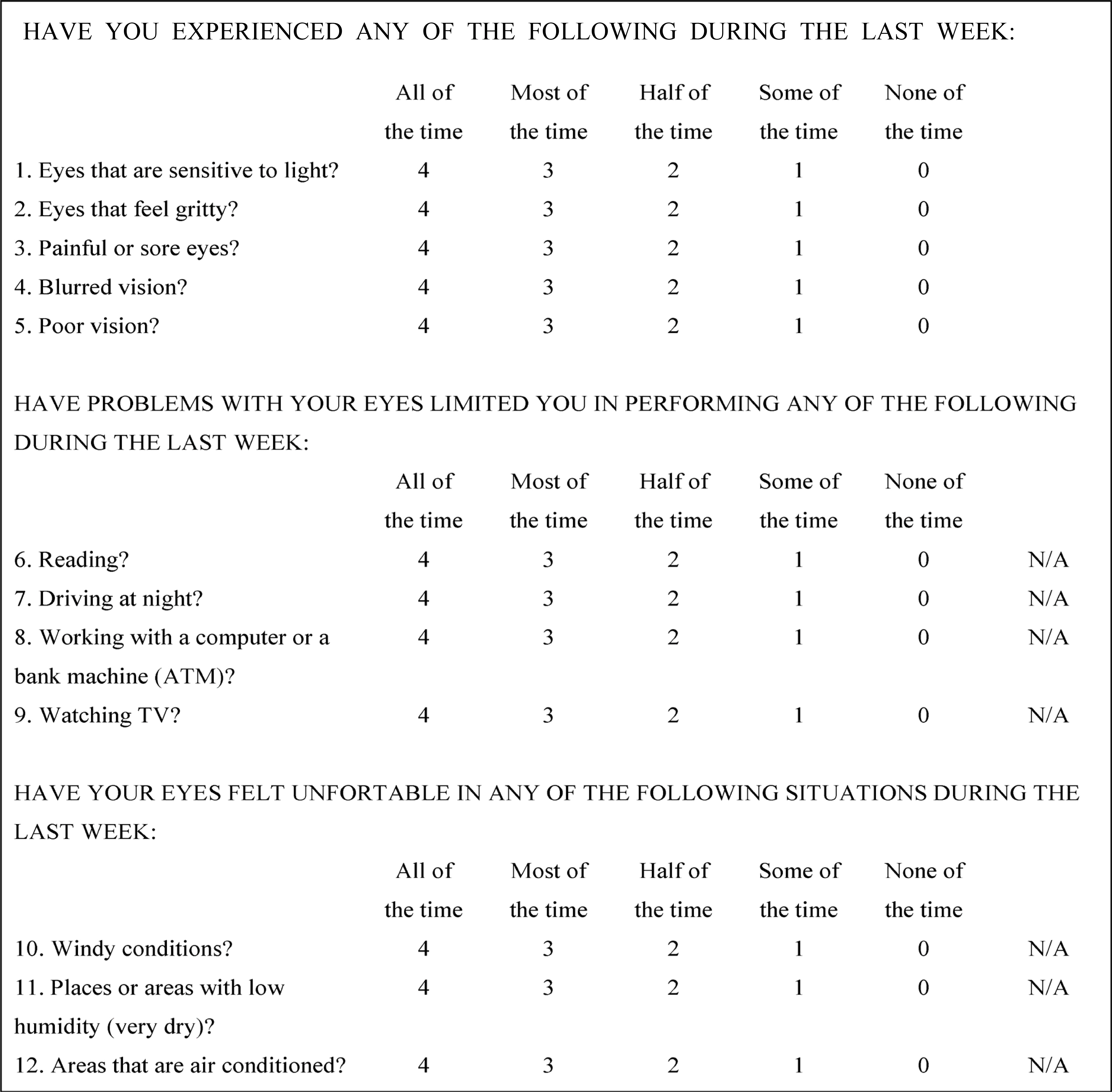
From patients diagnosed with mild dry eye syndrome, Restasis(R) was administered to 46 eyes of 23 patients. The clinical parameters and symptoms were checked over a period of six months. The clinical parameters evaluated were the symptoms of dry eye syndrome using an OSDI scoring scale, the Schirmer test, and tear break-up time.
RESULTS
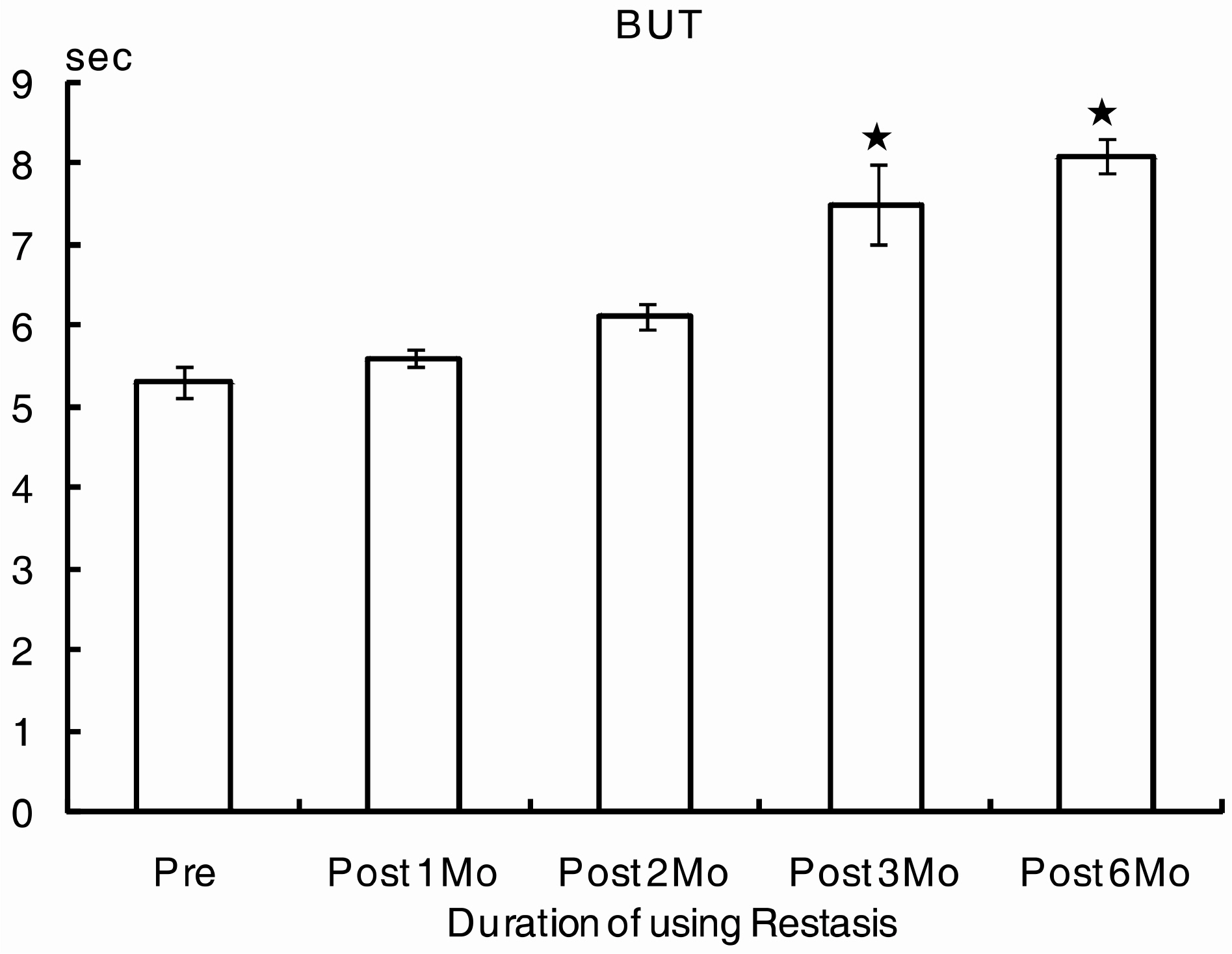
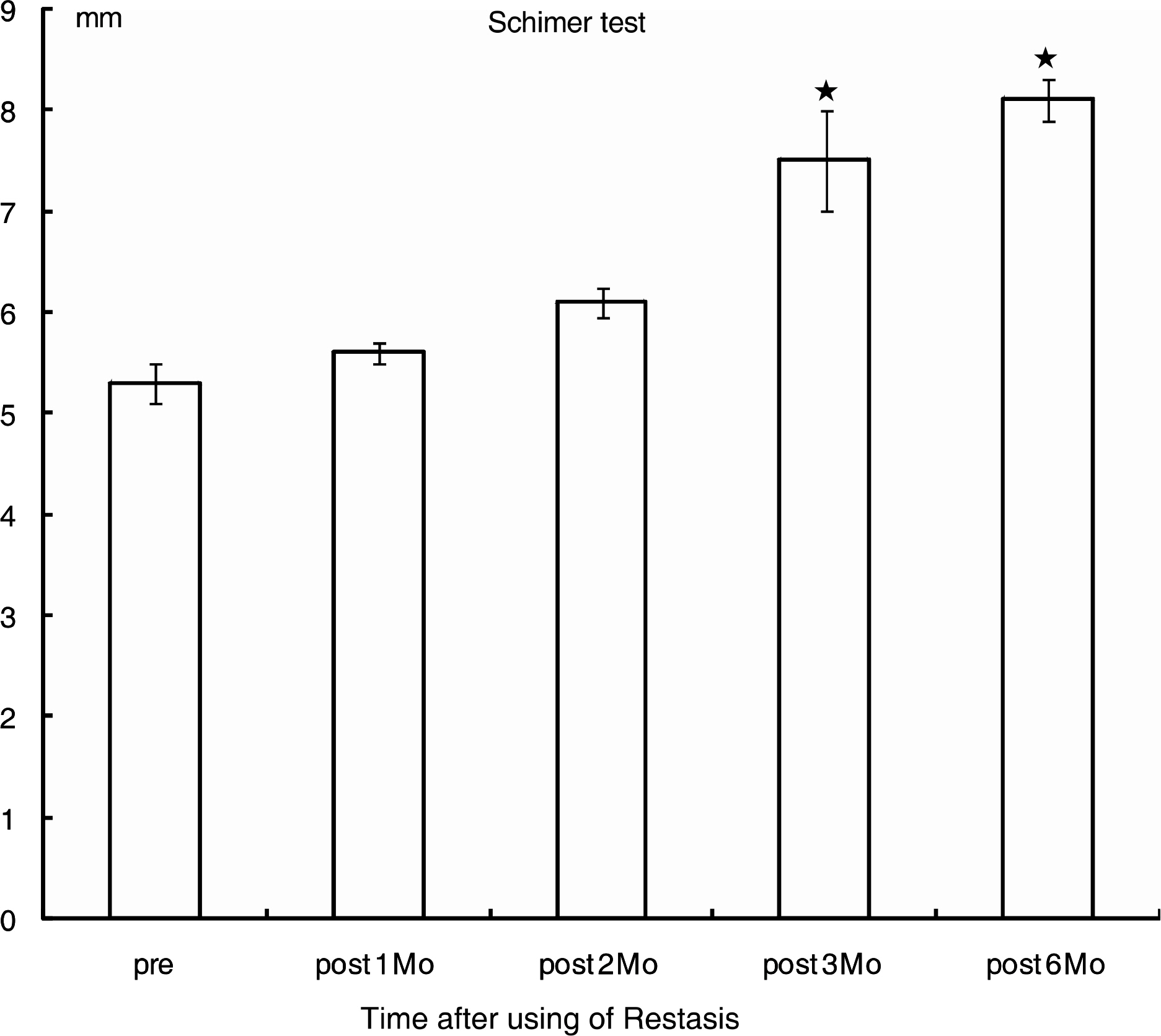
Eight male patients and 15 female patients were included in the study. The mean age was 50.5 years. Before treatment, the values for the OSDI score, BUT, and Schirmer test were 32.3, 5.3 mm, and 8.1 seconds, respectively. After treatment, the OSDI score, BUT, and Schirmer test were 22.9, 8.1 mm, and 12.1 seconds at six months, respectively. The subjective parameter (OSDI score) improved two months after treatment (p=0.003), and the objective parameters (BUT, Schirmer test) improved three months after treatment (p=0.03, p=0.04, respectively).
CONCLUSIONS
In the present study, Restasis(R) increased tear secretion and improved clinical symptoms of patients with mild dry eye.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Kunert KS, Tisdale AS, Stern ME, et al. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch Ophthalmol. 2000; 118:1489–96.2. Perry HD, Donnenfeld ED. Topical 0.05% cyclosporine A in the treatment of the dry eye. Expert Opin Pharmacother. 2004; 5:2099–107.3. Brignole F, Pisella PJ, de Saint Jean M, et al. Flow cytometric analysis of inflammatory markers in KCS: 6-month treatment with topical cyclosporine A. Invest Ophthalmol Vis Sci. 2001; 42:90–5.4. Wilson SE, Stulting RD. Agrement of physician treatment practices with the international task force guidelines for diagnosis and treatment of dry eye disease. Cornea. 2007; 26:284–9.5. O'Brien PD, Collum LM. Dry eye: Diagnosis and current treatment strategies. Curr Allergy Asthma Rep. 2004; 4:314–9.6. Stern ME, Pflugfelder SC. Inflammation in dry eye. Ocul Surf. 2004; 2:124–30.
Article7. Dogru M, Tsubota K. New insights into the diagnosis and treatment of dry eye. Ocul Surf. 2004; 2:59–75.
Article8. Hong S, Kim T, Chung SH, et al. Recurrence after topical nonpre-served methylprednisolone therapy for keratoconjunctivitis sicca in Sjogren's syndrome. J Ocul Pharmacol Ther. 2007; 23:78–82.9. Borel JF, Baumann G, Chapman I, et al. In vivo pharmacological effects of cyclosporin and some analogues. Adv Pharmacol. 1996; 35:115–246.10. Laibovitz RA, Solch S, Andriano K, et al. Pilot trial of cyclosporine 1% ophthalmic ointment in the treatment of keratoconjunctivitis sicca. Cornea. 1993; 12:315–23.
Article11. Power WJ, Mullaney P, Farrel M, Collum LM. Effect of topical cyclosporin A on conjunctival T cells in patients with secondary Sjongren's syndrome. Cornea. 1993; 12:507–11.12. Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease. Ophthalmology. 2000; 107:967–74.
Article13. Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. Ophthalmology. 2000; 107:631–9.14. Wilson SE, Perry HD. Long-term resolution of chronic dry eye symptoms and signs after topical cyclosporine treatment. Ophthalmology. 2007; 114:76–9.
Article15. Barber LD, Pflugfelder SC, Tauber J, Foulks GN. Phase III safty evaluation of cyclosporine 0.1% ophthalmic emulsion Administered twice daily to dry eye disease patients for up to 3 years. Ophthalmology. 2005; 112:1790–4.16. Perry HD, Solomon R, Donnenfeld ED, et al. Evaluation of topical cyclosporine for the treatment of dry eye disease. Arch Ophthalmol. 2008; 126:1046–50.
Article17. Byun YJ, Kim TI, Seo KY. The short-term effect of topical cyclosporine A 0.05% in various ocular surface disorder. J Korean Ophthalmol Soc. 2008; 49:401–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Effect of Cyclosporine 0.05% Eye Drops in Dry Eye Syndrome Patients
- Assessment of the Compliance with 0.1% Cyclosporine A in Dry-Eye Patients with Sjögren's Syndrome
- The Effect of Topical Cyclosporine 0.05% on Tear Osmolarity for Dry Eye Syndrome
- Effects of Carbomer Eye Gels on the Ocular Surface in Dry Eye Patients
- Bacterial Conjuntival Flora in Dry Eye Patients