J Korean Ophthalmol Soc.
2008 Oct;49(10):1583-1588.
Clinical Effect of Cyclosporine 0.05% Eye Drops in Dry Eye Syndrome Patients
- Affiliations
-
- 1Department of Ophthalmology and Visual Science, St. Mary's Hospital, The College of Medicine, The Catholic University of Korea, Seoul, Korea. eyedoc@catholic.ac.kr
Abstract
- PURPOSE
We conducted a study to evaluate the change of tear secretion and symptoms in the patients with dry eye syndrome after using Restasis(R) (Cyclosporine 0.05% ophthalmic emulsion, Allergan Inc., U.S.A.).
METHODS
We randomly selected 39 patients from newly or previously diagnosed dry eye syndrome patients and administered Restasis(R) to them. We checked their clinical parameters and symptoms over a period of 3 months. The clinical parameters evaluated were type I and type II Schirmer tests and tear break-up time, and the symptoms of dry eye syndrome were classified into pain, itching, foreign body sensation, blurred vision, and photophobia using a scoring scale for symptoms of 0 to 5. The results were analyzed with a Mann-Whitney test (P-values <0.05 was considered statistically significant).
RESULTS
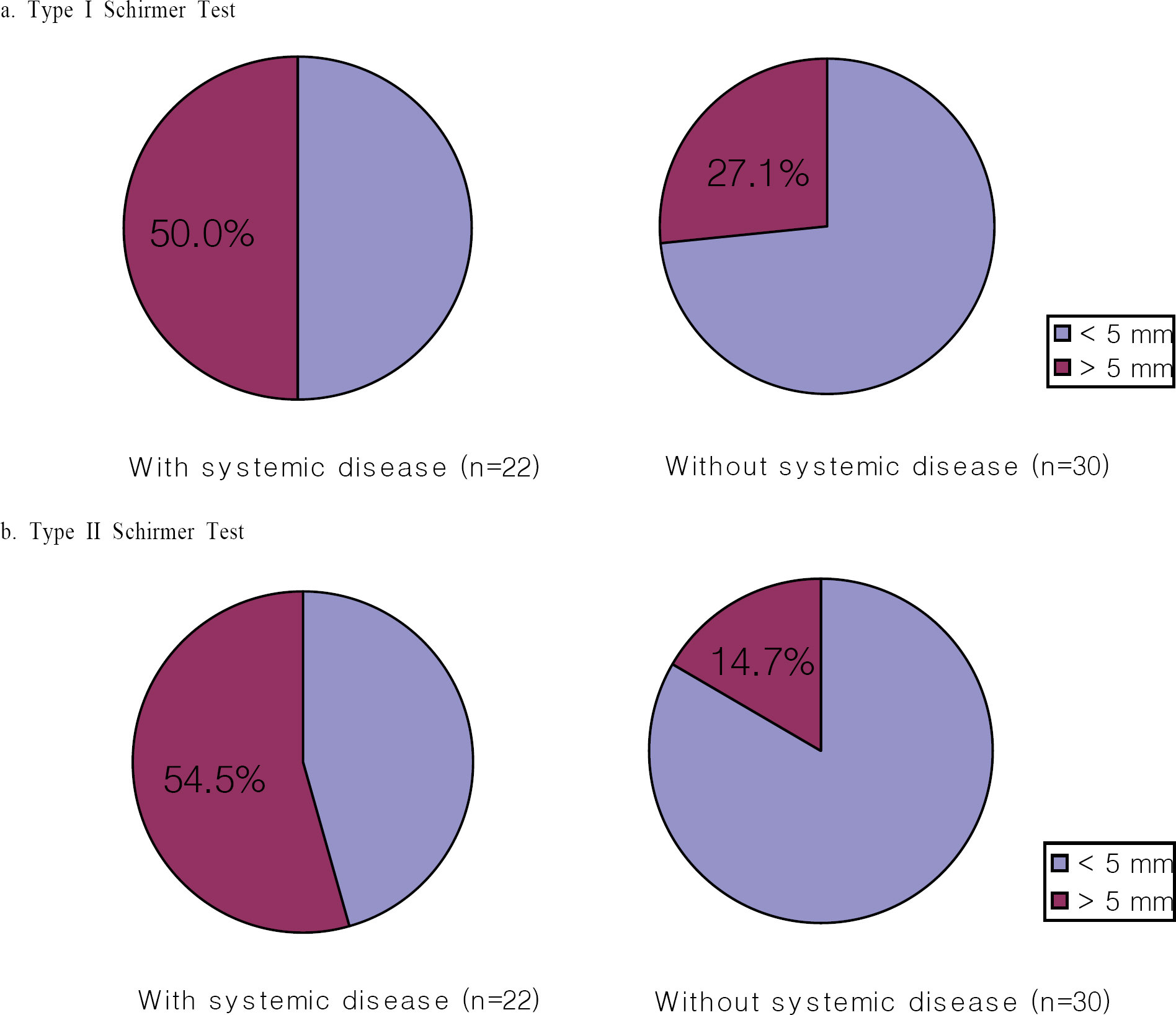
For 26 of 39 patients (52 eyes) on whom all tests were carried out for 3 months, there was a significant improvement after 3 months in the type I Schirmer test, type II Schirmer test, and tear break-up time (P=0.012, 0.009, 0.001, respectively). Only 14 patients completed the questionnaire for scoring of symptoms. After using Restasis(R), foreign body sensation only improved (P=0.010).
CONCLUSIONS
In our study, tear secretion was increased by Restasis(R), and a greater increase in tear secretion was seen in patients with systemic disease than in patients without systemic disease. Additional patients need to be evaluated and longer-term studies need to be performed to confirm our results.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Pflugfelder SC, Tseng SC, Sanabria O. . Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea. 1998; 17:38–56.
Article2. Darsun D, Kim MC, Solomon A, Pflugfelder SC. Treatment of recalcitrant recurrent corneal epithelial erosions with inhibitors of matrix metalloproteinases-9, doxycycline and corticosteroids. Am J Ophthalmol. 2001; 132:8–13.3. Marsh P, Pflugfelder SC. Topical nonpreserved methylpredni solone-plus therapy of keratoconjunctivitis sicca in Sjogren's syndrome. Ophthalmology. 1999; 106:509–12.4. Gündüz K, Ozdemir O. Topical cyclosporin treatment of keratoconjunctivitis sicca in secondary Sjogren's syndrome. Acta Ophthalmol. 1994; 72:438–42.5. Tsubota K, Goto E, Fujita H. . Treatment of dry eye by autologous serum application in Sjogren's syndrome. Br J Ophthalmol. 1999; 83:390–5.
Article6. Pflugfelder SC, Solomon A, Stern ME. The diagnosis and management of dry eye: a twenty-five-year review. Cornea. 2000; 19:644–9.7. Stern ME, Beuerman RW, Fox RI. . The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998; 17:584–9.8. Stern ME, Gao J, Siemasko KF. . The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004; 78:409–16.
Article9. Smith VA, Rishmawi H, Hussein H, Easty DL. Tear film MMP accumulation and corneal disease. Br J Ophthalmol. 2001; 85:147–53.
Article10. Solomon A, Dursun D, Liu Z. . Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001; 42:2283–92.11. Balaram M. . Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjögren syndrome. Invest Ophthalmol Vis Sci. 2002; 43:1004–11.12. Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002; 120:330–7.
Article13. Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. Ophthalmology. 2000; 107:631–9.14. Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporine A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. Cyclosporine A Phase 2 Study Group. Ophthalmology. 2000; 107:967–74.15. Brignole F, Pisella PJ. . Flow cytometric analysis of inflammatory markers in KCS: 6-month treatment with topical cyclosporine A. Invest Ophthalmol Vis Sci. 2001; 42:90–5.16. Kunert KS, Tisdale AS, Stern ME. . Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch Ophthalmol. 2000; 118:1489–96.17. Turner K, Pflugfelder SC, Ji Z. . Interleukin-6 levels in the conjunctival epithelium of patients with dry eye disease treated with cyclosporine ophthalmic emulsion. Cornea. 2000; 19:492–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the Effects of 0.05% and 0.1% Cyclosporine for Dry Eye Syndrome Patients after Cataract Surgery
- Assessment of the Compliance with 0.1% Cyclosporine A in Dry-Eye Patients with Sjögren's Syndrome
- Effect of Combined Treatment with Cyclosporine A and Cord Serum for Dry Eye Associated with Graft-Versus-Host-Disease
- The Effect of Topical Cyclosporine 0.05% on Dry Eye after Cataract Surgery
- Cinical Effect of Restasis(R) Eye Drops in Mild Dry Eye Syndrome