J Korean Ophthalmol Soc.
2009 Jun;50(6):911-918. 10.3341/jkos.2009.50.6.911.
Comparison of Vitreolytic Effect in Rabbit Eyes: Plasmin, Hyaluronidase, and Their Mixtures
- Affiliations
-
- 1Department of Ophthalmology, KyungHee University Hospital, Seoul, Korea. hwkwak@khmc.or.kr
- 2Department of Ophthalmology, Inje University College of Medicine, Seoul Paik Hospital, Seoul, Korea.
- 3Department of Ophthalmology, Chung-Ang University College of Medicine, Seoul, Korea.
- KMID: 2212370
- DOI: http://doi.org/10.3341/jkos.2009.50.6.911
Abstract
-
PURPOSE: The aim of the present study was to quantify and compare the vitreolytic effect of plasmin, hyaluronidase, and a combination of the two.
METHODS
Thirty-six rabbits were randomized into 3 groups: (A) twelve rabbits had an intravitreal injection of plasmin 1 U with hyaluronidase 10 U/0.1 mL into the right eye, (B) twelve rabbits had an injection of plasmin alone (1 U/0.1 mL), and (C) twelve rabbits had an injection of hyaluronidase alone (10 U/0.1 mL). The left eye of each rabbit was used as control, which was injected with 0.1 mL phosphate buffered saline (PBS). The eyes were enucleated 1 hour and 24 hours after injection. The volume of fluid-type vitreous and gel-type vitreous was measured with a micropipette using the melting point as the difference. Statistical analysis was performed and light microscopy was used to assess potential damage to the retinal tissue.
RESULTS
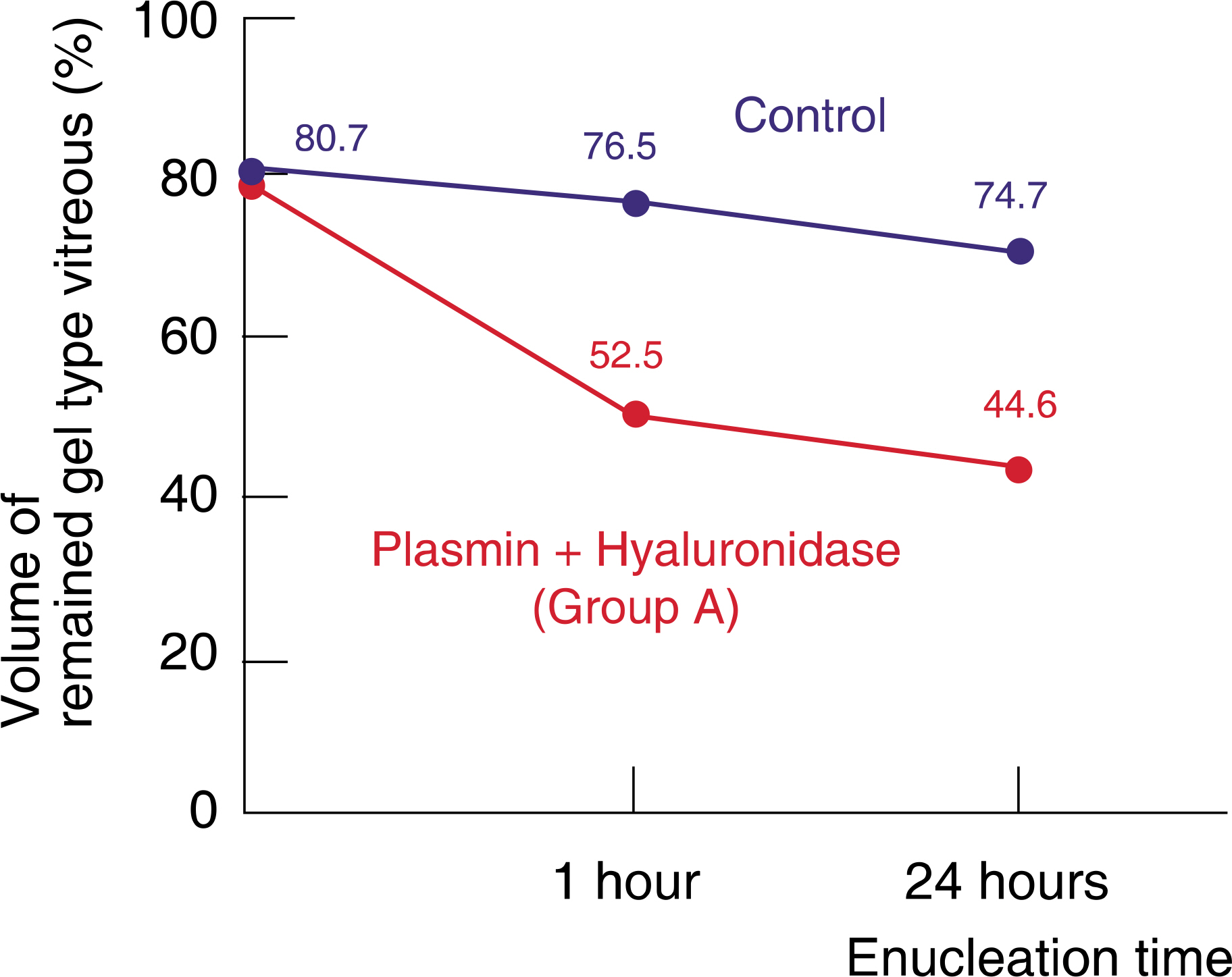
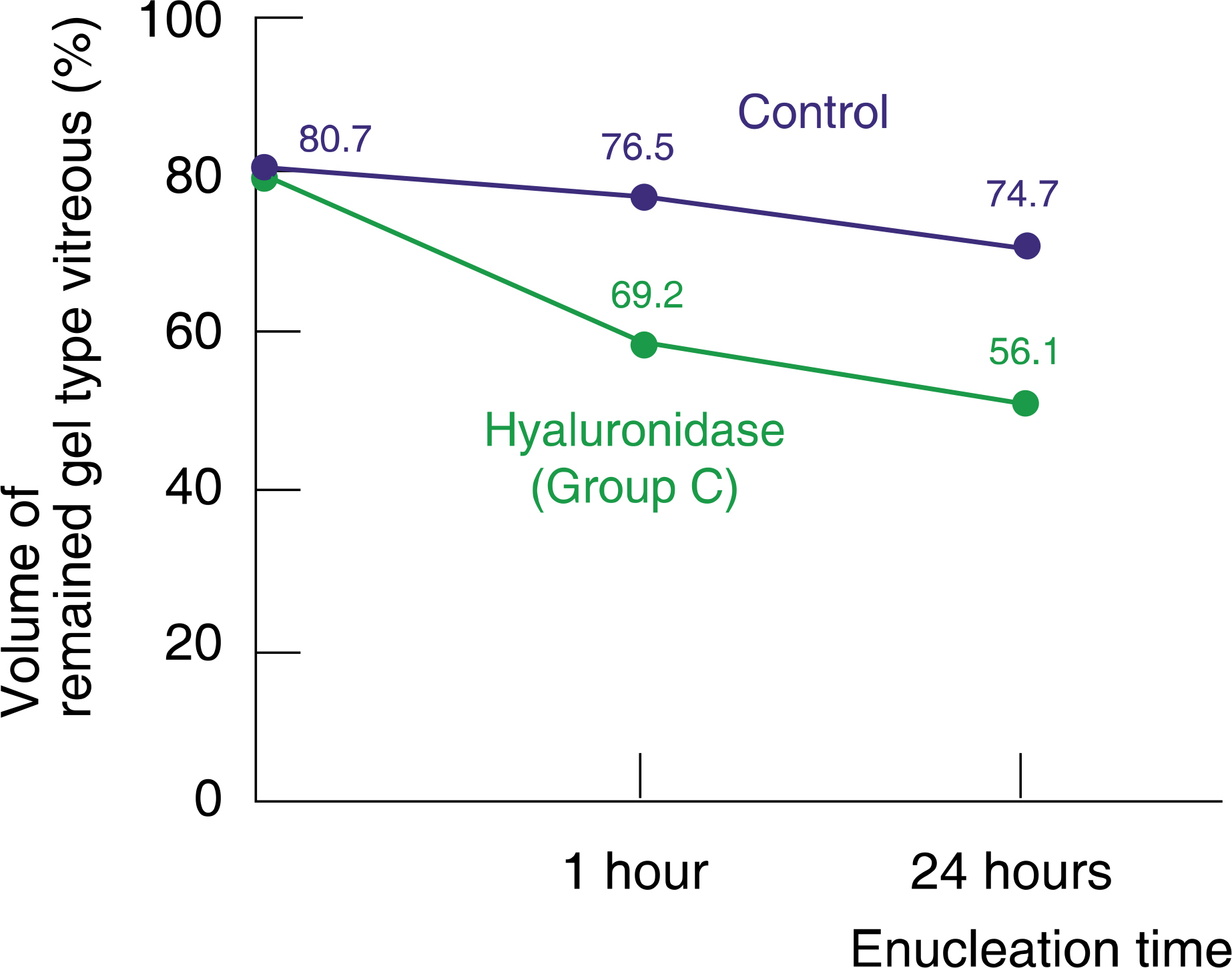
The volume of remaining gel-type vitreous was measured as 52.5%, 60.3%, 59.2%, and 76.5% after 1 hour enucleation and as 44.6%, 56.7%, 56.1%, and 74.7%, after 24 hours enucleation in group A, B, C, and control group, respectively. Group A, B, and C showed statistically significant differences against the control group. Group A (plasmin with hyaluronidase) showed less remaining gel-type vitreous volume than a single injection of plasmin or hyaluronidase alone.
CONCLUSIONS
Intravitreal injection of plasmin with hyaluronidase showed more vitreolytic effect than a single injection of plasmin or hyaluronidase alone. The enzyme may be useful in liquefying the vitreous, and may be a useful biochemical adjunct to vitrectomy.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Efficacy of Autologous Plasmin-Assisted Vitrectomy for Rhegmatogenous Retinal Detachment
Chaerin Park, Sun Ho Lee, Jang Won Heo, Hum Chung
J Korean Ophthalmol Soc. 2011;52(7):825-831. doi: 10.3341/jkos.2011.52.7.825.
Reference
-
References
1. Sebag J. Pharmacologic vitreolysis. Retina. 1998; 18:1–3.
Article2. Sebag J. Pharmacologic vitreolysis brewing? Retina. 2002; 22:1–3.3. Hayreh SS, Jonas JB. Posterior Vitreous detachment: Clinical correlations. Ophthalmologica. 2004; 218:333–43.
Article4. Sebag J. Diabetic vitreopathy. Ophthalmology. 1996; 103:205–6.
Article5. Takahashi MK, Hikichi T, Akiba J, et al. Role of the vitreous and macular edema in branch retinal vein occlusion. Ophthalmic Surg Lasers. 1997; 28:294–9.6. Sonoda K, Sakamoto T, Enaida H, et al. Residual vitreous cortex after surgical posterior vitreous separation visualized by intravitreal triamcinolone acetonide. Ophthalmology. 2004; 111:226–30.7. Asami T, Terasaki H, Kachi S, et al. Ultrastructure of inter-nallimiting membrane removed during plasmin assisted vitrectomy from eyes with diabetic macular edema. Ophthalmology. 2004; 111:231–7.8. Verstraeten TC, Chapman C, Hartzer M, et al. Pharmacologic inductionof posterior vitreous detachment in the rabbit. Arch Ophthalmol. 1993; 111:849–54.9. Tanaka M, Qui H. Pharmacological vitrectomy. Semin Ophthalmol. 2000; 15:51–61.
Article10. Bishop PN. Structural macromolecules and supramolecular organisation of the vitreous gel. Prog-Retin Eye Res. 2000; 19:323–44.
Article11. Kuppermann BD, Thomas EL, de Smet MD, Grillone LR. Safety results of two phaseⅢ trials of an intravitreous injection of highly purified ovine hyaluronidase (Vitrase) for the management of vitreous hemorrhage. Am J Ophthalmol. 2005; 140:585–97.12. Kim NJ, Yu HG, Yu YS, Chung H. Long term effect of plasmin on the vitreolysis in the rabbit eyes. Korean J Ophthalmology. 2004; 18:35–40.13. Wang ZL, Zhang X, Xu X, et al. PVD following plasmin but nothyaluronidase: implication for combination pharmacologic vitreolysis. Retina. 2005; 25:38–43.14. Staubach F, Nober V, Janknecht P. Enzyme-assisted vitrectomy in enucleated pig eyes: a comparison of hyaluronidase, chondroitinase, and plasmin. Curr Eye Res. 2004; 29:261–8.
Article15. Wang ZL, Zhang X, Xu X, et al. Pharmacologic vitreolysis combining the two enzymes plasmin and hyaluronidase. Retina. 2005; 25:674–5.16. Howard M, Sen HA, Capoor S, et al. Measurement of adenosine concentration in aqueous and vitreous. Invest Ophthalmol Vis Sci. 1998; 39:1942–6.17. Kim DS, Moon SW, Yu SY, Kwak HW. The structure of the internal limiting membrane removed by vitrectomy using tissue plasminogen activator. J Korean Ophthalmol Soc. 2008; 49:917–24.
Article18. Gottlieb JL, Antoszyk AN, Hatchell DL, Saloupis P. The safety of intravitreal hyaluronidase. A clinical and histological study. Invest Ophthalmol Vis Sci. 1990; 31:2345–52.19. Harooni M, McMillan T, Refojo M. Efficacy and safety of enzymatic posterior vitreous detachment by intravitreal injection of hyaluronidase. Retina. 1998; 18:16–22.
Article20. Kang SW, Hyung S, Choi MY, Lee J. Induction of vitreolysis and vitreous detachment with hyaluronidase and perfluoropropane gas. Korean J Ophthalmol. 1995; 9:69–78.
Article21. Li X, Shi X, Fan J. Posterior vitreous detachment with plasmin in the isolated human eye. Graefes Arch Clin Exp Ophthalmol. 2002; 240:56–62.
Article22. Wang F, Wang Z, Sun X, et al. Safety and efficacy of dispase and plasmin in pharmacologic vitreolysis. Invest Ophthalmol Vis Sci. 2004; 45:3286–90.
Article23. Gandorfer A, Putz E, Welge-Lussen U, et al. Ultrastructure of the vitreoretinal interface following plasmin assisted vitrectomy. Br J Ophthalmol. 2001; 85:6–10.
Article24. Gandorfer A, priglinger S, Schebitz K, et al. Vitreoretinal morphology of plasmin-treated human eyes. Am J Ophthalmol. 2001; 133:156–9.25. Gandorfer A, Rohleder M, Sethi C, et al. Posterior vitreous detachment induced by microplasmin. Invest Ophthalmol Vis Sci. 2004; 45:641–7.
Article26. Plantner JJ, Smine A, Quinn TA. Matrix metalloproteinases and metalloproteinase inhibitors in human interphotoreceptor matrix and vitreous. Curr Eye Res. 1998; 17:132–40.
Article27. Baramova EN, Bajou K, Remacle A, et al. Involvement of PA/plasminsystem in the processing of pro-MMP-9 and in the second step of pro-MMP-2 activation. FEBS Lett. 1997; 157:157–62.28. Ramos-DeSimone N, Hahn-Dantona E, Sipley J, et al. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/ stromelysin-1 cascade enhances tumor cell invasion. J Biol Chem. 1999; 274:13066–76.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long-term Effect of Plasmin on the Vitreolysis in Rabbit Eyes
- Hyaluronidase: An overview of its properties, applications, and side effects
- The Effect of Alpha-chymotrypsin, Trypsin and Hyaluronidase on the Viscosity of the Rabbit's Vitreous Humor
- Induction of vitreolysis and vitreous detachment with hyaluronidase and perfluoropropane gas
- Delayed Allergic Reaction to Secondary Administrated Epidural Hyaluronidase