J Periodontal Implant Sci.
2010 Jun;40(3):125-131. 10.5051/jpis.2010.40.3.125.
Investigation of bone formation using calcium phosphate glass cement in beagle dogs
- Affiliations
-
- 1Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. shchoi726@yuhs.ac
- 2Department and Research Institute of Dental Biomaterials and Bioengineering, Yonsei University College of Dentistry, Seoul, Korea.
- KMID: 2212139
- DOI: http://doi.org/10.5051/jpis.2010.40.3.125
Abstract
- PURPOSE
Among available biomaterials, bioceramics have drawn special interest due to their bioactivity and the possibility of tailoring their composition. The degradation rate and formulation of bioceramics can be altered to mimic the compositions of the mineral phase of bone. The aim of this study was to investigate the bone formation effect of amorphous calcium phosphate glass cement (CPGC) synthesized by a melting and quenching process.
METHODS
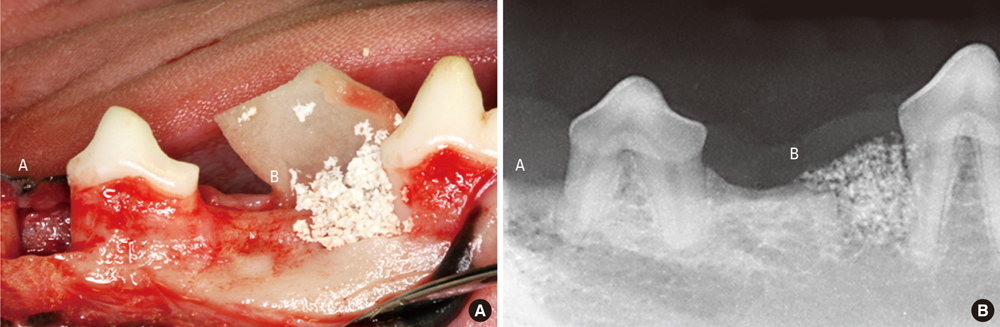
In five male beagle dogs, 4 x 4 mm 1-wall intrabony defects were created bilaterally at the mesial or distal aspect of the mandibular second and fourth premolars. Each of the four defects was divided according to graft materials: CPGC with collagen membrane (CM), biphasic calcium phosphate (BCP) with CM, CM alone, or a surgical flap operation only. The dogs were sacrificed 8 weeks post-surgery, and block sections of the defects were collected for histologic and histometric analysis.
RESULTS
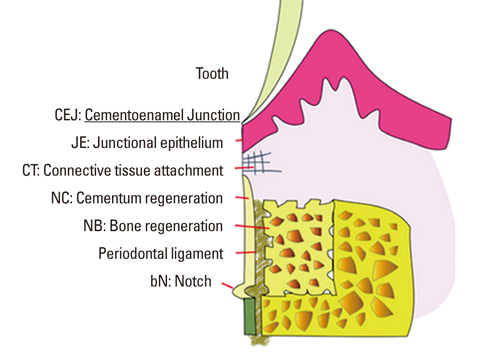
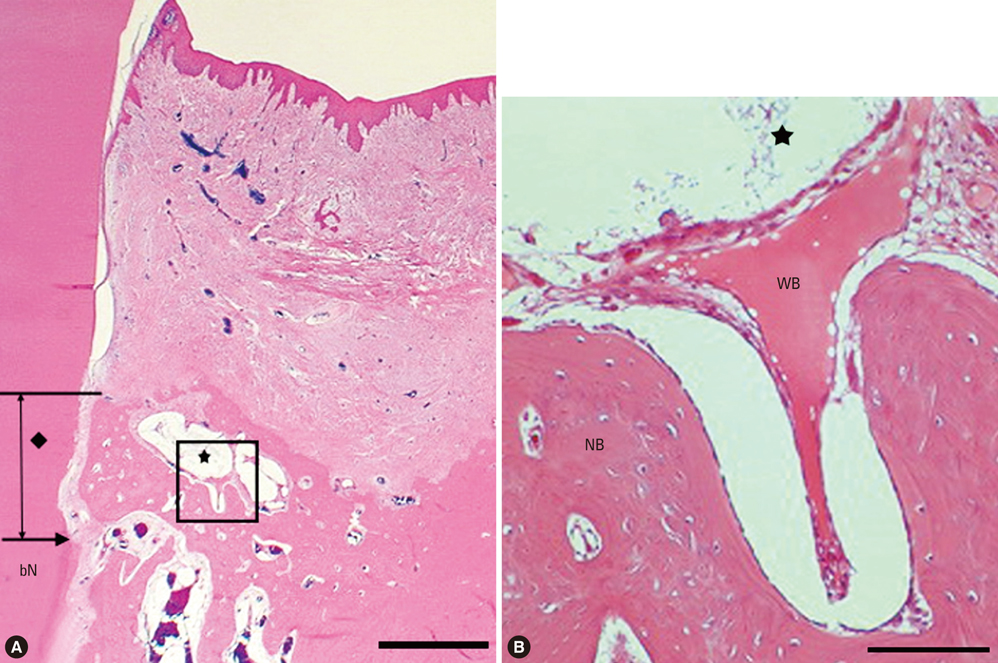
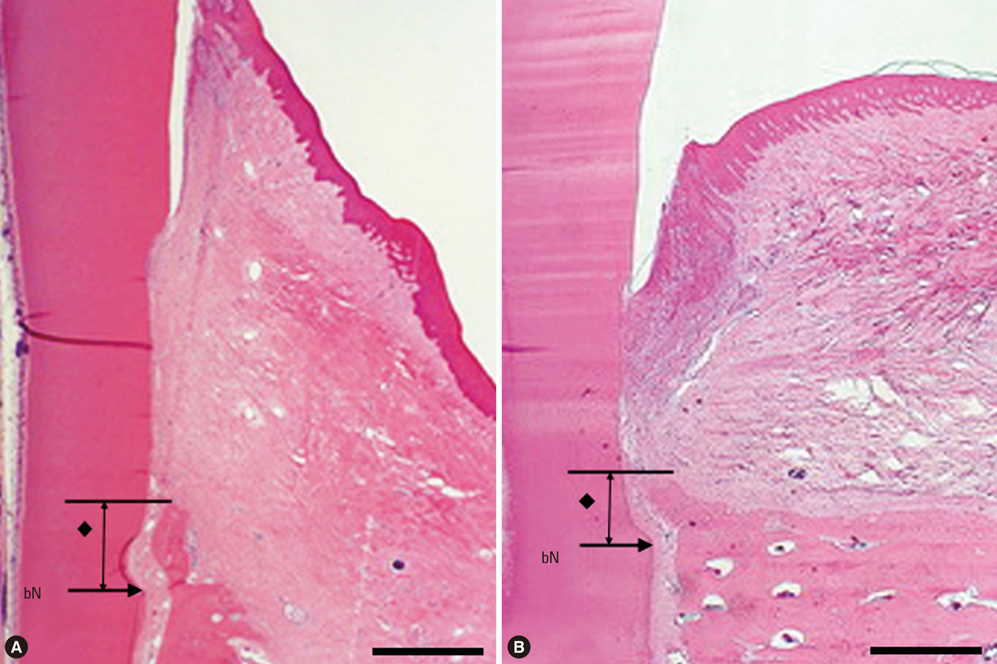
There were significant differences in bone formation and cementum regeneration between the experimental and control groups. In particular, the CPGC and BCP groups showed greater bone formation than the CM and control groups.
CONCLUSIONS
In conclusion, CPGC was replaced rapidly with an abundant volume of new bone; CPGC also contributed slightly to regeneration of the periodontal apparatus.
Keyword
MeSH Terms
-
Animals
Bicuspid
Biocompatible Materials
Bone Substitutes
Calcium
Calcium Phosphates
Collagen
Dental Cementum
Dogs
Freezing
Glass
Humans
Hydrazines
Hydroxyapatites
Male
Membranes
Osteogenesis
Regeneration
Surgical Flaps
Transplants
Biocompatible Materials
Bone Substitutes
Calcium
Calcium Phosphates
Collagen
Hydrazines
Hydroxyapatites
Figure
Reference
-
1. Parikh SN. Bone graft substitutes in modern orthopedics. Orthopedics. 2002. 25:1301–1309.
Article2. Ahlmann E, Patzakis M, Roidis N, Shepherd L, Holtom P. Comparison of anterior and posterior iliac crest bone grafts in terms of harvest-site morbidity and functional outcomes. J Bone Joint Surg Am. 2002. 84A:716–720.
Article3. Kurashina K, Kurita H, Wu Q, Ohtsuka A, Kobayashi H. Ectopic osteogenesis with biphasic ceramics of hydroxyapatite and tricalcium phosphate in rabbits. Biomaterials. 2002. 23:407–412.
Article4. Jarcho M, Kay JF, Gumaer KI, Doremus RH, Drobeck HP. Tissue, cellular and subcellular events at a bone-ceramic hydroxylapatite interface. J Bioeng. 1977. 1:79–92.5. Rabalais ML Jr, Yukna RA, Mayer ET. Evaluation of durapatite ceramic as an alloplastic implant in periodontal osseous defects. I. Initial six-month results. J Periodontol. 1981. 52:680–689.
Article6. Gao H, Tan T, Wang D. Effect of composition on the release kinetics of phosphate controlled release glasses in aqueous medium. J Control Release. 2004. 96:21–28.
Article7. Bagambisa FB, Joos U, Schilli W. Interaction of osteogenic cells with hydroxylapatite implant materials in vitro and in vivo. Int J Oral Maxillofac Implants. 1990. 5:217–226.8. Kim YK, Yun PY, Lim SC, Kim SG, Lee HJ, Ong JL. Clinical evaluations of OSTEON as a new alloplastic material in sinus bone grafting and its effect on bone healing. J Biomed Mater Res B Appl Biomater. 2008. 86:270–277.
Article9. Hosono H, Abe Y. Porous glass-ceramics composed of a titanium phosphate crystal skeleton: a review. J Non-Cryst Solids. 1995. 190:185–197.
Article10. Lee BH, Kim MC, Kim KN, LeGeros RZ, Lee YK. Biodegradable bone cement using calcium phosphate glass. Key Eng Mater. 2006. 309-311:861–864.
Article11. Nery EB, Eslami A, Van Swol RL. Biphasic calcium phosphate ceramic combined with fibrillar collagen with and without citric acid conditioning in the treatment of periodontal osseous defects. J Periodontol. 1990. 61:166–172.
Article12. Bohner M. Physical and chemical aspects of calcium phosphates used in spinal surgery. Eur Spine J. 2001. 10:Suppl 2. S114–S121.
Article13. Bunker BC, Arnold GW, Wilder JA. Phosphate glass dissolution in aqueous solutions. J Non-Cryst Solids. 1984. 64:291–316.
Article14. Murphy KG, Gunsolley JC. Guided tissue regeneration for the treatment of periodontal intrabony and furcation defects. A systematic review. Ann Periodontol. 2003. 8:266–302.
Article15. Dias AG, Lopes MA, Gibson JR, Santos JD. In vitro degradation studies of calcium phosphate glass ceramics prepared by controlled crystallization. J Non-Cryst Solids. 2003. 330:81–89.
Article16. Maus U, Andereya S, Gravius S, Ohnsorge JA, Niedhart C, Siebert CH. BMP-2 incorporated in a tricalcium phosphate bone substitute enhances bone remodeling in sheep. J Biomater Appl. 2008. 22:559–576.
Article17. Wolff KD, Swaid S, Nolte D, Bockmann RA, Holzle F, Muller-Mai C. Degradable injectable bone cement in maxillofacial surgery: indications and clinical experience in 27 patients. J Craniomaxillofac Surg. 2004. 32:71–79.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects on the Tissue Reaction Using GI Cement in the Maxillary Grade II Furcation in the Beagle Dogs
- Novel Calcium Phosphate Glass for Hard-Tissue Regeneration
- The Biological Effects of Calcium Phosphate Coated Implant for Osseointegration in Beagle Dogs
- Histologic findings of three-wall intrabony defects around dental implants using different grafting materials in beagle dogs
- The effects of noncrystalline calcium phosphate glass on the healing of 1-wall intrabony defects in beagle dogs