J Periodontal Implant Sci.
2012 Feb;42(1):13-19. 10.5051/jpis.2012.42.1.13.
In situ dental implant installation after decontamination in a previously peri-implant diseased site: a pilot study
- Affiliations
-
- 1Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. shchoi726@yuhs.ac
- 2Division of Periodontolgy, Department of Dentistry, National Health Insurance Corporation Ilsan Hospital, Goyang, Korea.
- KMID: 2212080
- DOI: http://doi.org/10.5051/jpis.2012.42.1.13
Abstract
- PURPOSE
The aim of this study was to examine whether a previous peri-implantitis site can affect osseointegration, by comparing implant placement at a site where peri-implantitis was present and at a normal bone site. A second aim of this study was to identify the tissue and bone reaction after treating the contaminated implant surface to determine the optimal treatment for peri-implant diseases.
METHODS
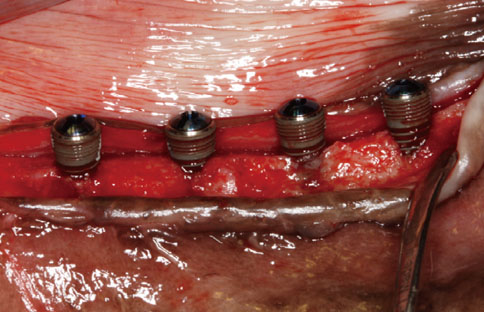
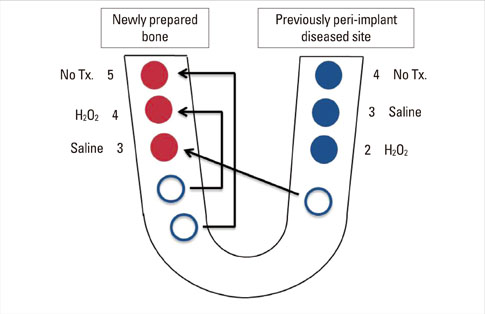
A peri-implant mucositis model for dogs was prepared to determine the optimal treatment option for peri-implant mucositis or peri-implantitis. The implants were inserted partially to a length of 6 mm. The upper 4 mm part of the dental implants was exposed to the oral environment. Simple exposure for 2 weeks contaminated the implant surface. After 2 weeks, the implants were divided into three groups: untreated, swabbed with saline, and swabbed with H2O2. Three implants from each group were placed to the full length in the same spot. The other three implants were placed fully into newly prepared bone. After eight weeks of healing, the animals were sacrificed. Ground sections, representing the mid-buccal-lingual plane, were prepared for histological analysis. The analysis was evaluated clinically and histometrically.
RESULTS
The untreated implants and H2O2-swabbed implants showed gingival inflammation. Only the saline-swabbed implant group showed re-osseointegration and no gingival inflammation. There was no difference in regeneration height or bone-to-implant contact between in situ implant placement and implant placement in the new bone site.
CONCLUSIONS
It can be concluded that cleaning with saline may be effective in implant decontamination. After implant surface decontamination, implant installation in a previous peri-implant diseased site may not interfere with osseointegration.
MeSH Terms
Figure
Reference
-
1. Wennerberg A, Albrektsson T. Current challenges in successful rehabilitation with oral implants. J Oral Rehabil. 2011. 38:286–294.
Article2. Lambert FE, Weber HP, Susarla SM, Belser UC, Gallucci GO. Descriptive analysis of implant and prosthodontic survival rates with fixed implant-supported rehabilitations in the edentulous maxilla. J Periodontol. 2009. 80:1220–1230.
Article3. Renvert S, Persson GR. Periodontitis as a potential risk factor for peri-implantitis. J Clin Periodontol. 2009. 36:Suppl 10. 9–14.
Article4. Mombelli A, van Oosten MA, Schurch E Jr, Land NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 1987. 2:145–151.
Article5. Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008. 35:8 Suppl. 286–291.
Article6. Lindhe J, Meyle J. Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008. 35:8 Suppl. 282–285.
Article7. Berglundh T, Persson L, Klinge B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J Clin Periodontol. 2002. 29:Suppl 3. 197–212.
Article8. Alcoforado GA, Rams TE, Feik D, Slots J. Microbial aspects of failing osseointegrated dental implants in humans. J Parodontol. 1991. 10:11–18.
Article9. Leonhardt A, Renvert S, Dahlén G. Microbial findings at failing implants. Clin Oral Implants Res. 1999. 10:339–345.
Article10. Schwarz F, Jepsen S, Herten M, Sager M, Rothamel D, Becker J. Influence of different treatment approaches on non-submerged and submerged healing of ligature induced peri-implantitis lesions: an experimental study in dogs. J Clin Periodontol. 2006. 33:584–595.
Article11. Schwarz F, Bieling K, Latz T, Nuesry E, Becker J. Healing of intrabony peri-implantitis defects following application of a nanocrystalline hydroxyapatite (Ostim) or a bovine-derived xenograft (Bio-Oss) in combination with a collagen membrane (Bio-Gide). A case series. J Clin Periodontol. 2006. 33:491–499.
Article12. Sculean A, Schwarz F, Becker J. Anti-infective therapy with an Er:YAG laser: influence on peri-implant healing. Expert Rev Med Devices. 2005. 2:267–276.
Article13. Schou S, Holmstrup P, Skovgaard LT, Stoltze K, Hjørting-Hansen E, Gundersen HJ. Autogenous bone graft and ePTFE membrane in the treatment of peri-implantitis. II. Stereologic and histologic observations in cynomolgus monkeys. Clin Oral Implants Res. 2003. 14:404–411.
Article14. Schou S, Holmstrup P, Jørgensen T, Skovgaard LT, Stoltze K, Hjørting-Hansen E, et al. Implant surface preparation in the surgical treatment of experimental peri-implantitis with autogenous bone graft and ePTFE membrane in cynomolgus monkeys. Clin Oral Implants Res. 2003. 14:412–422.
Article15. Persson LG, Araújo MG, Berglundh T, Gröndahl K, Lindhe J. Resolution of peri-implantitis following treatment. An experimental study in the dog. Clin Oral Implants Res. 1999. 10:195–203.16. Persson LG, Berglundh T, Lindhe J, Sennerby L. Re-osseointegration after treatment of peri-implantitis at different implant surfaces. An experimental study in the dog. Clin Oral Implants Res. 2001. 12:595–603.17. Alhag M, Renvert S, Polyzois I, Claffey N. Re-osseointegration on rough implant surfaces previously coated with bacterial biofilm: an experimental study in the dog. Clin Oral Implants Res. 2008. 19:182–187.18. Parlar A, Bosshardt DD, Cetiner D, Schafroth D, Unsal B, Haytaç C, et al. Effects of decontamination and implant surface characteristics on re-osseointegration following treatment of peri-implantitis. Clin Oral Implants Res. 2009. 20:391–399.19. Kolonidis SG, Renvert S, Hämmerle CH, Lang NP, Harris D, Claffey N. Osseointegration on implant surfaces previously contaminated with plaque. An experimental study in the dog. Clin Oral Implants Res. 2003. 14:373–380.
Article20. Schou S, Holmstrup P, Reibel J, Juhl M, Hjørting-Hansen E, Kornman KS. Ligature-induced marginal inflammation around osseointegrated implants and ankylosed teeth: stereologic and histologic observations in cynomolgus monkeys (Macaca fascicularis). J Periodontol. 1993. 64:529–537.
Article21. Heitz-Mayfield LJ, Lang NP. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontol 2000. 2010. 53:167–181.
Article22. Grunder U, Hürzeler MB, Schüpbach P, Strub JR. Treatment of ligature-induced peri-implantitis using guided tissue regeneration: a clinical and histologic study in the beagle dog. Int J Oral Maxillofac Implants. 1993. 8:282–293.
Article23. Jovanovic SA, Kenney EB, Carranza FA Jr, Donath K. The regenerative potential of plaque-induced peri-implant bone defects treated by a submerged membrane technique: an experimental study. Int J Oral Maxillofac Implants. 1993. 8:13–18.
Article24. Mohamed S, Polyzois I, Renvert S, Claffey N. Effect of surface contamination on osseointegration of dental implants surrounded by circumferential bone defects. Clin Oral Implants Res. 2010. 21:513–519.
Article25. Renvert S, Samuelsson E, Lindahl C, Persson GR. Mechanical non-surgical treatment of peri-implantitis: a double-blind randomized longitudinal clinical study. I: clinical results. J Clin Periodontol. 2009. 36:604–609.
Article26. Persson LG, Ericsson I, Berglundh T, Lindhe J. Osseintegration following treatment of peri-implantitis and replacement of implant components. An experimental study in the dog. J Clin Periodontol. 2001. 28:258–263.
Article27. Albouy JP, Abrahamsson I, Persson LG, Berglundh T. Implant surface characteristics influence the outcome of treatment of peri-implantitis: an experimental study in dogs. J Clin Periodontol. 2011. 38:58–64.
Article28. Persson GR, Samuelsson E, Lindahl C, Renvert S. Mechanical non-surgical treatment of peri-implantitis: a single-blinded randomized longitudinal clinical study. II. Microbiological results. J Clin Periodontol. 2010. 37:563–573.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Unusual bone regeneration following resective surgery and decontamination of peri-implantitis: a 6-year follow-up
- Laser therapy in peri-implantitis treatment: literature review
- Regenerative procedure using rotary titanium brush for surface decontamination of peri-implantitis: 3 cases with a 2-year follow-up
- Management of peri-implantitis associated with tear-like implant fracture: case reports
- Risk factors of peri-implantitis: a narrative review