J Periodontal Implant Sci.
2015 Oct;45(5):169-177. 10.5051/jpis.2015.45.5.169.
Peri-implant crevicular fluid levels of cathepsin-K, RANKL, and OPG around standard, short, and mini dental implants after prosthodontic loading
- Affiliations
-
- 1Department of Periodontology, Necmettin Erbakan University Faculty of Dentistry, Konya, Turkey. drraifalan17@gmail.com
- 2Department of Periodontology, Selcuk University Faculty of Dentistry, Konya, Turkey.
- 3Department of Biochemistry, Selcuk University Faculty of Dentistry, Konya, Turkey.
- KMID: 2212005
- DOI: http://doi.org/10.5051/jpis.2015.45.5.169
Abstract
- PURPOSE
Despite the high success rates of endosseous dental implants, their placement is restricted according to the height and volume of bone available. The use of short or mini dental implants could be one way to overcome this limitation. Thus, this study aimed to compare standard, short, and mini dental implants with regard to associated clinical parameters and peri-implant crevicular fluid (PICF) levels of cathepsin -K (CTSK), RANK ligand (RANKL), and osteoprotegerin (OPG), after prosthodontic loading.
METHODS
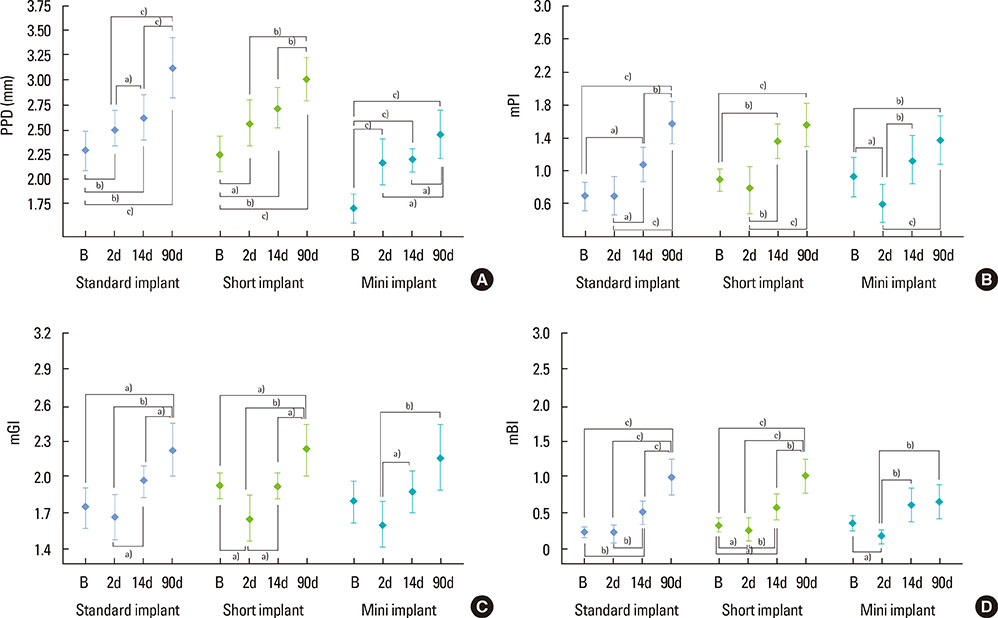
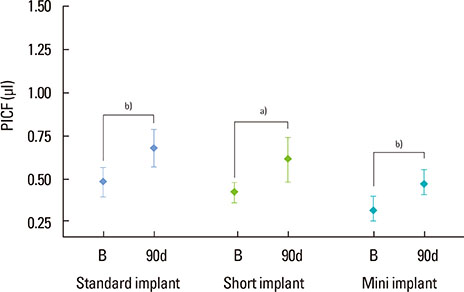
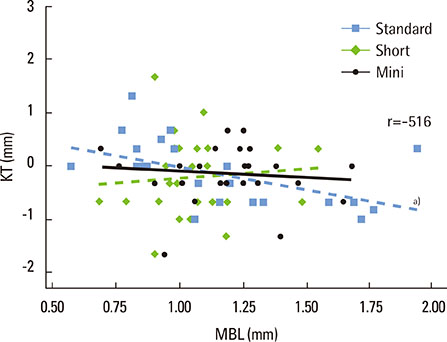
A total of 78 non-submerged implants (Euroteknika, Aesthetica+2, Sallanches, France) were installed in 30 subjects (13 male, 17 female; range, 26-62 years) who visited the clinic of the Periodontology Department, Faculty of Dentistry, Selcuk University. Sampling and measurements were performed on the loading date (baseline) and 2, 14, and 90 days after loading. Assessment of the peri-implant status for the implant sites was performed using the pocket probing depth (PPD), modified plaque index, modified gingival index, modified sulcular bleeding index, and radiographic signs of bone loss. PICF samples collected from each implant were evaluated for CTSK, RANKL, and OPG levels using the ELISA method. Keratinized tissue and marginal bone loss (MBL) were also noted.
RESULTS
Clinical parameters statistically significantly increased in each group but did not show statistical differences between groups without PPD. Although implant groups showed a higher MBL in the upper jaw, only the standard dental group demonstrated a statistically significant difference. At 90 days, the OPG: sRANKL ratio and total amounts of CTSK for each group did not differ from baseline.
CONCLUSIONS
Within the limitations of this study, both short and mini dental implants were achieving the same outcomes as the standard dental implants in the early period after loading.
MeSH Terms
Figure
Reference
-
1. De Boever AL, Quirynen M, Coucke W, Theuniers G, De Boever JA. Clinical and radiographic study of implant treatment outcome in periodontally susceptible and non-susceptible patients: a prospective long-term study. Clin Oral Implants Res. 2009; 20:1341–1350.
Article2. Lee DZ, Chen ST, Darby IB. Maxillary sinus floor elevation and grafting with deproteinized bovine bone mineral: a clinical and histomorphometric study. Clin Oral Implants Res. 2012; 23:918–924.
Article3. Lai HC, Si MS, Zhuang LF, Shen H, Liu YL, Wismeijer D. Long-term outcomes of short dental implants supporting single crowns in posterior region: a clinical retrospective study of 5-10 years. Clin Oral Implants Res. 2013; 24:230–237.
Article4. Renouard F, Nisand D. Impact of implant length and diameter on survival rates. Clin Oral Implants Res. 2006; 17:Suppl 2. 35–51.
Article5. Gentile MA, Chuang SK, Dodson TB. Survival estimates and risk factors for failure with 6 × 5.7-mm implants. Int J Oral Maxillofac Implants. 2005; 20:930–937.6. Hansson S. The implant neck: smooth or provided with retention elements. A biomechanical approach. Clin Oral Implants Res. 1999; 10:394–405.
Article7. Hasan I, Heinemann F, Aitlahrach M, Bourauel C. Biomechanical finite element analysis of small diameter and short dental implant. Biomed Tech Berl. 2010; 55:341–350.
Article8. Bhardwaj S, Prabhuji ML. Comparative volumetric and clinical evaluation of peri-implant sulcular fluid and gingival crevicular fluid. J Periodontal Implant Sci. 2013; 43:233–242.
Article9. Kajale AM, Mehta DS. Interleukin-1β level in peri-implant crevicular fluid and its correlation with the clinical and radiographic parameters. J Indian Soc Periodontol. 2014; 18:220–225.
Article10. Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999; 20:345–357.
Article11. Bartold PM, Cantley MD, Haynes DR. Mechanisms and control of pathologic bone loss in periodontitis. Periodontol 2000. 2010; 53:55–69.
Article12. Bostanci N, Ilgenli T, Emingil G, Afacan B, Han B, Töz H, et al. Gingival crevicular fluid levels of RANKL and OPG in periodontal diseases: implications of their relative ratio. J Clin Periodontol. 2007; 34:370–376.
Article13. Li Z, Hou WS, Brömme D. Collagenolytic activity of cathepsin K is specifically modulated by cartilage-resident chondroitin sulfates. Biochemistry. 2000; 39:529–536.
Article14. Mogi M, Otogoto J. Expression of cathepsin-K in gingival crevicular fluid of patients with periodontitis. Arch Oral Biol. 2007; 52:894–898.
Article15. Yamalik N, Günday S, Uysal S, Kilinç K, Karabulut E, Tözüm TF. Analysis of cathepsin-K activity at tooth and dental implant sites and the potential of this enzyme in reflecting alveolar bone loss. J Periodontol. 2012; 83:498–505.
Article16. Takamiya AS, Goiato MC, Gennari Filho H. Effect of smoking on the survival of dental implants. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014; 158:650–653.
Article17. Mombelli A, van Oosten MA, Schurch E Jr, Land NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 1987; 2:145–151.
Article18. Löe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963; 21:533–551.
Article19. Algraffee H, Borumandi F, Cascarini L. Peri-implantitis. Br J Oral Maxillofac Surg. 2012; 50:689–694.
Article20. Schrott AR, Jimenez M, Hwang JW, Fiorellini J, Weber HP. Five-year evaluation of the influence of keratinized mucosa on peri-implant soft-tissue health and stability around implants supporting full-arch mandibular fixed prostheses. Clin Oral Implants Res. 2009; 20:1170–1177.
Article21. Manz MC. Factors associated with radiographic vertical bone loss around implants placed in a clinical study. Ann Periodontol. 2000; 5:137–151.
Article22. Rakic M, Struillou X, Petkovic-Curcin A, Matic S, Canullo L, Sanz M, et al. Estimation of bone loss biomarkers as a diagnostic tool for peri-implantitis. J Periodontol. 2014; 85:1566–1574.
Article23. Monov G, Strbac GD, Baron M, Kandler B, Watzek G, Gruber R. Soluble RANKL in crevicular fluid of dental implants: a pilot study. Clin Implant Dent Relat Res. 2006; 8:135–141.
Article24. Arikan F, Buduneli N, Kütükçüler N. Osteoprotegerin levels in peri-implant crevicular fluid. Clin Oral Implants Res. 2008; 19:283–288.
Article25. Lang NP, Berglundh T. Working Group 4 of Seventh European Workshop on Periodontology. Periimplant diseases: where are we now?--Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011; 38:Suppl 11. 178–181.
Article26. Lu HK, Chen YL, Chang HC, Li CL, Kuo MY. Identification of the osteoprotegerin/receptor activator of nuclear factor-kappa B ligand system in gingival crevicular fluid and tissue of patients with chronic periodontitis. J Periodontal Res. 2006; 41:354–360.
Article27. Yamalik N, Günday S, Kilinc K, Karabulut E, Berker E, Tözüm TF. Analysis of cathepsin-K levels in biologic fluids from healthy or diseased natural teeth and dental implants. Int J Oral Maxillofac Implants. 2011; 26:991–997.28. Strbac GD, Monov G, Cei S, Kandler B, Watzek G, Gruber R. Cathepsin K levels in the crevicular fluid of dental implants: a pilot study. J Clin Periodontol. 2006; 33:302–308.
Article29. Misch CE, Perel ML, Wang HL, Sammartino G, Galindo-Moreno P, Trisi P, et al. Implant success, survival, and failure: the International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant Dent. 2008; 17:5–15.
Article30. Jemt T, Lekholm U. Implant treatment in edentulous maxillae: a 5-year follow-up report on patients with different degrees of jaw resorption. Int J Oral Maxillofac Implants. 1995; 10:303–311.31. Tinsley D, Watson CJ, Ogden AR. A survey of U.K. centres on implant failures. J Oral Rehabil. 1999; 26:14–18.
Article32. Koldsland OC, Scheie AA, Aass AM. Prevalence of implant loss and the influence of associated factors. J Periodontol. 2009; 80:1069–1075.
Article33. Todescan S, Lavigne S, Kelekis-Cholakis A. Guidance for the maintenance care of dental implants: clinical review. J Can Dent Assoc. 2012; 78:c107.34. Roos-Jansåker AM, Lindahl C, Renvert H, Renvert S. Nine- to fourteen-year follow-up of implant treatment. Part I: implant loss and associations to various factors. J Clin Periodontol. 2006; 33:283–289.
Article35. Bouri A Jr, Bissada N, Al-Zahrani MS, Faddoul F, Nouneh I. Width of keratinized gingiva and the health status of the supporting tissues around dental implants. Int J Oral Maxillofac Implants. 2008; 23:323–326.36. Martin W, Lewis E, Nicol A. Local risk factors for implant therapy. Int J Oral Maxillofac Implants. 2009; 24:28–38.37. Heitz-Mayfield LJ. Peri-implant diseases: diagnosis and risk indicators. J Clin Periodontol. 2008; 35:292–304.
Article38. Hämmerle CH, Glauser R. Clinical evaluation of dental implant treatment. Periodontol 2000. 2004; 34:230–239.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Assessment of interleukin-1beta and interleukin-6 in the crevicular fluid around healthy implants, implants with peri-implantitis, and healthy teeth: a cross-sectional study
- Prevalence and risk factors of peri-implant mucositis and peri-implantitis after at least 7 years of loading
- Comparative volumetric and clinical evaluation of peri-implant sulcular fluid and gingival crevicular fluid
- Hyaluronic acid reduces inflammation and crevicular fluid IL-1β concentrations in peri-implantitis: a randomized controlled clinical trial
- Analysis of time to failure of orthodontic mini-implants after insertion or loading