J Nutr Health.
2015 Oct;48(5):381-389. 10.4163/jnh.2015.48.5.381.
High fructose and high fat diet increased bone volume of trabecular and cortical bone in growing female rats
- Affiliations
-
- 1Department of Medical Nutrition, Kyung Hee University, Gyeonggi 17104, Korea. ypark@khu.ac.kr
- 2Research Institute of Medical Nutrition, Kyung Hee University, Seoul 02447, Korea.
- KMID: 2209872
- DOI: http://doi.org/10.4163/jnh.2015.48.5.381
Abstract
- PURPOSE
The objective of this study was to investigate the effects of a high fructose and fat diet on bone growth and maturation in growing female rats.
METHODS
Three-week-old female SD rats were randomly assigned to four experimental groups; the control group (CON: fed control diet based on AIN-93G, n = 8); the high-fructose diet group (HFrc: fed control diet with 30% fructose, n = 8); the high-fat diet group (Hfat: fed control diet with 45 kcal% fat, n = 8); and the high-fat diet plus high fructose group (HFrc + HFat: fed diets 45 kcal% fat with 30% fructose, n = 8). Each group was assigned their respective diets for the remaining eight weeks. Bone-related parameters (bone mineral density (BMD) and structural parameters, osteocalcin (OC), deoxypyridinoline (DPD)) and morphologic changes of kidney were analyzed at the end of the experiment.
RESULTS
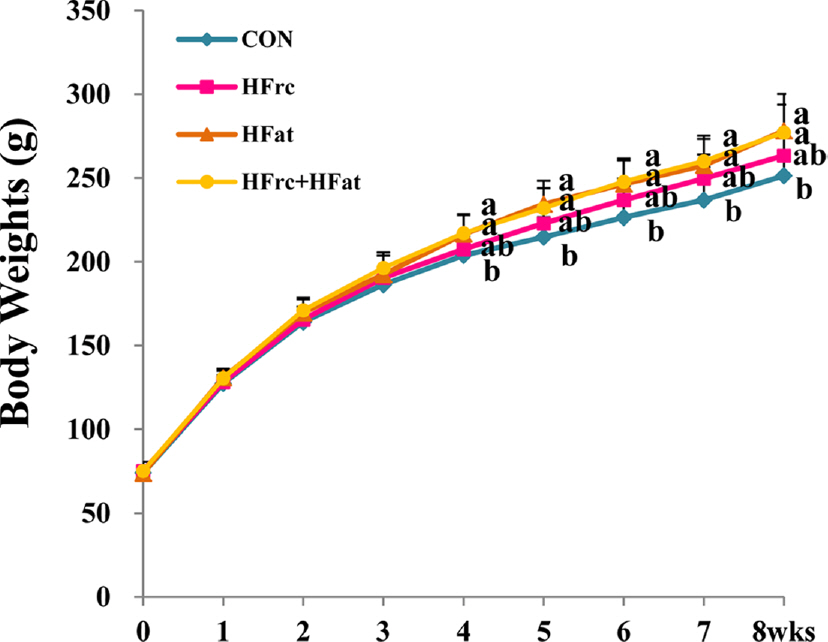
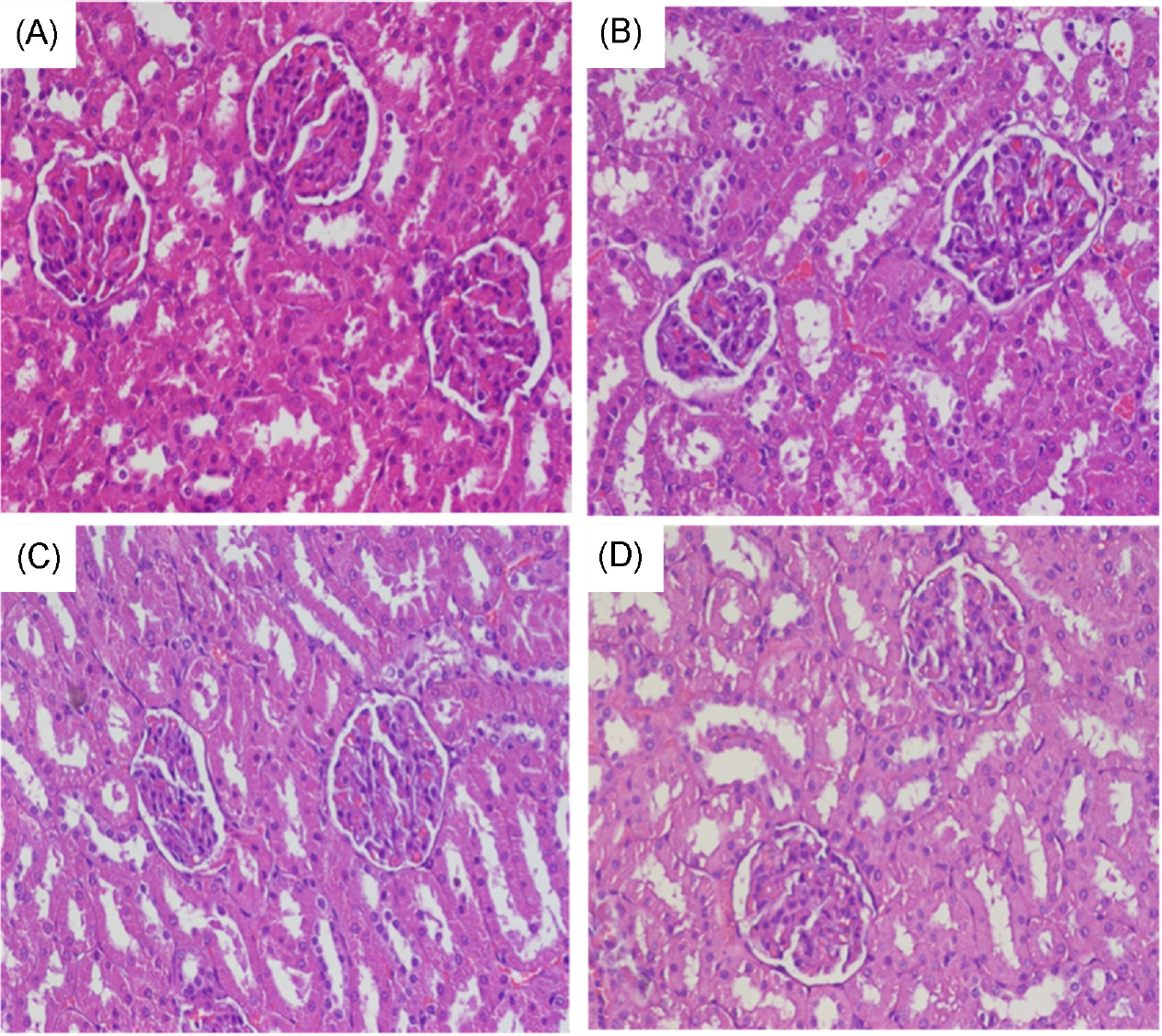
Final body weights and weight gain were higher in the HFat and HFrc + HFat groups and showed higher tendency in the HFrc group compared with those of the CON group (p < 0.05); however, no significant difference in caloric intake was observed among the four experimental groups. The serum OC levels of the HFrc and HFrc + HFat groups were lower than those of the CON and HFat groups (p < 0.05). Urinary levels of DPD did not differ among the experimental groups. BV/TV and Tb.N of trabecular bone were higher in the HFrc + HFat group and showed a higher tendency in the HFrc group than those of the CON and HFat groups (p < 0.05). Tb.Pf of trabecular bone were lower in the HFrc + HFat group than those in the CON and HFat groups (p < 0.05). However, no difference in trabecular BMD was observed among the experimental groups. Cortical bone volume was higher in the HFat and HFrc + HFat groups than in the CON and HFrc groups (p < 0.05). No morphology change in kidney was observed among the experimental groups.
CONCLUSION
Our study suggests that 8 weeks of high-fructose and high fat intake could improve the bone quality (Structural parameters) of trabecular and cortical bone of tibia in growing female rats.
MeSH Terms
Figure
Reference
-
1.Kim SD., Moon HK., Park JS., Yang HR., Yi YJ., Han EJ., Lee YC., Shin GY., Kim JH., Chae YZ. The content of macrominerals in beverages, liquid teas, and liquid coffees. J Korean Soc Food Sci Nutr. 2012. 41(8):1134–1143.
Article2.Kim SD., Moon HK., Park JS., Lee YC., Shin GY., Jo HB., Kim BS., Kim JH., Chae YZ. Macromineral intake in non-alcoholic beverages for children and adolescents: using the fourth Korea National Health and Nutrition Examination Survey (KNHANES ?, 2007-2009). Korean J Nutr. 2013. 46(1):50–60.3.Chung SJ., Kim JH., Lee JS., Lee DH., Kim SH., Yu CH. A suggestion to develop a nutrition policy on food and nutrition labeling and education systems for fast food and carbonated soft drinks in Korea. Korean J Nutr. 2004. 37(5):394–405.4.Ruff JS., Hugentobler SA., Suchy AK., Sosa MM., Tanner RE., Hite ME., Morrison LC., Gieng SH., Shigenaga MK., Potts WK. Compared to sucrose, previous consumption of fructose and glucose monosaccharides reduces survival and fitness of female mice. J Nutr. 2015. 145(3):434–441.
Article5.Chung M., Ma J., Patel K., Berger S., Lau J., Lichtenstein AH. Fructose, high-fructose corn syrup, sucrose, and nonalcoholic fatty liver disease or indexes of liver health: a systematic review and meta-analysis. Am J Clin Nutr. 2014. 100(3):833–849.
Article6.Stanhope KL., Bremer AA., Medici V., Nakajima K., Ito Y., Nakano T., Chen G., Fong TH., Lee V., Menorca RI., Keim NL., Havel PJ. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab. 2011. 96(10):E1596–E1605.
Article7.Douard V., Asgerally A., Sabbagh Y., Sugiura S., Shapses SA., Casi-rola D., Ferraris RP. Dietary fructose inhibits intestinal calcium absorption and induces vitamin D insufficiency in CKD. J Am Soc Nephrol. 2010. 21(2):261–271.
Article8.Palanisamy N., Viswanathan P., Anuradha CV. Effect of genistein, a soy isoflavone, on whole body insulin sensitivity and renal damage induced by a high-fructose diet. Ren Fail. 2008. 30(6):645–654.9.Dissard R., Klein J., Caubet C., Breuil B., Siwy J., Hoffman J., Sicard L., Ducassé L., Rascalou S., Payre B., Buléon M., Mullen W., Mischak H., Tack I., Bascands JL., Buffin-Meyer B., Schanstra JP. Long term metabolic syndrome induced by a high fat high fructose diet leads to minimal renal injury in C57BL/6 mice. PLoS One. 2013. 8(10):e76703.
Article10.Whiting SJ., Vatanparast H., Baxter-Jones A., Faulkner RA., Mir-wald R., Bailey DA. Factors that affect bone mineral accrual in the adolescent growth spurt. J Nutr. 2004. 134(3):696S–700S.
Article11.Singh D., Sanyal S., Chattopadhyay N. The role of estrogen in bone growth and formation: changes at puberty. Cell Health Cytoskelet. 2011. 3(1):2–12.
Article12.Kang BS., Park MS., Cho YS., Lee JW. Beverage consumption and related factors among adolescents in the Chungnam urban area. Korean J Community Nutr. 2006. 11(4):469–478.13.Song MJ., An EM., Shon HS., Kim SB., Cha YS. A study on the status of beverage consumption of the middle school students in Jeonju. Korean J Community Nutr. 2005. 10(2):174–182.14.Bass EF., Baile CA., Lewis RD., Giraudo SQ. Bone quality and strength are greater in growing male rats fed fructose compared with glucose. Nutr Res. 2013. 33(12):1063–1071.
Article15.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008. 3(Suppl 3):S131-S139.
Article16.Seibel MJ. Biochemical markers of bone turnover: part I: biochemistry and variability. Clin Biochem Rev. 2005. 26(4):97–122.17.Vasikaran SD. Utility of biochemical markers of bone turnover and bone mineral density in management of osteoporosis. Crit Rev Clin Lab Sci. 2008. 45(2):221–258.
Article18.Tsanzi E., Light HR., Tou JC. The effect of feeding different sugar-sweetened beverages to growing female Sprague-Dawley rats on bone mass and strength. Bone. 2008. 42(5):960–968.
Article19.Felice JI., Gangoiti MV., Molinuevo MS., McCarthy AD., Cortizo AM. Effects of a metabolic syndrome induced by a fructose-rich diet on bone metabolism in rats. Metabolism. 2014. 63(2):296–305.
Article20.Li YQ., Xing XH., Wang H., Weng XL., Yu SB., Dong GY. Dose-dependent effects of genistein on bone homeostasis in rats' mandibular subchondral bone. Acta Pharmacol Sin. 2012. 33(1):66–74.
Article21.Piekarz AV., Ward WE. Effect of neonatal exposure to genistein on bone metabolism in mice at adulthood. Pediatr Res. 2007. 61(1):48–53.
Article22.Seidlová-Wuttke D., Jarry H., Jäger Y., Wuttke W. Bone development in female rats maintained with soy-free or soy-containing food as determined by computer-assisted tomography and serum bone markers. J Bone Miner Metab. 2008. 26(4):321–327.
Article23.Kretowicz M., Johnson RJ., Ishimoto T., Nakagawa T., Manitius J. The impact of fructose on renal function and blood pressure. Int J Nephrol. 2011. 2011:315879.
Article24.Mohamed Salih S., Nallasamy P., Muniyandi P., Periyasami V., Carani Venkatraman A. Genistein improves liver function and attenuates non-alcoholic fatty liver disease in a rat model of insulin resistance. J Diabetes. 2009. 1(4):278–287.
Article25.Nakagawa T., Hu H., Zharikov S., Tuttle KR., Short RA., Glushakova O., Ouyang X., Feig DI., Block ER., Herrera-Acosta J., Patel JM., Johnson RJ. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006. 290(3):F625–F631.
Article26.Shih CC., Lin CH., Lin WL., Wu JB. Momordica charantia extract on insulin resistance and the skeletal muscle GLUT4 protein in fructose-fed rats. J Ethnopharmacol. 2009. 123(1):82–90.
Article27.Hamrick MW., Pennington C., Newton D., Xie D., Isales C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone. 2004. 34(3):376–383.
Article28.Douard V., Sabbagh Y., Lee J., Patel C., Kemp FW., Bogden JD., Lin S., Ferraris RP. Excessive fructose intake causes 1,25-(OH)(2)D (3)-dependent inhibition of intestinal and renal calcium transport in growing rats. Am J Physiol Endocrinol Metab. 2013. 304(12):E1303–E1313.29.Douard V., Suzuki T., Sabbagh Y., Lee J., Shapses S., Lin S., Ferraris RP. Dietary fructose inhibits lactation-induced adaptations in rat 1,25-(OH)?D? synthesis and calcium transport. FASEB J. 2012. 26(2):707–721.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Femoral bone structure in Otsuka Long-Evans Tokushima Fatty rats
- Measurement of Bone Mineral Density in Children with Normal Growth and Development
- Differential Effect of Vitamin K and Vitamin D Supplementation on Bone Mass in Young Rats Fed Normal or Low Calcium Diet
- A study on the trabecular change of Femur according to 17beta-Estradiol Dosage in Ovariectomized Rat
- Growth and Age-Related Abnormalities in Cortical Structure and Fracture Risk