Endocrinol Metab.
2015 Dec;30(4):419-428. 10.3803/EnM.2015.30.4.419.
Growth and Age-Related Abnormalities in Cortical Structure and Fracture Risk
- Affiliations
-
- 1Division of Endocrinology, Department of Medicine, Austin Health, University of Melbourne, Melbourne, Australia. egos@unimelb.edu.au
- KMID: 2169648
- DOI: http://doi.org/10.3803/EnM.2015.30.4.419
Abstract
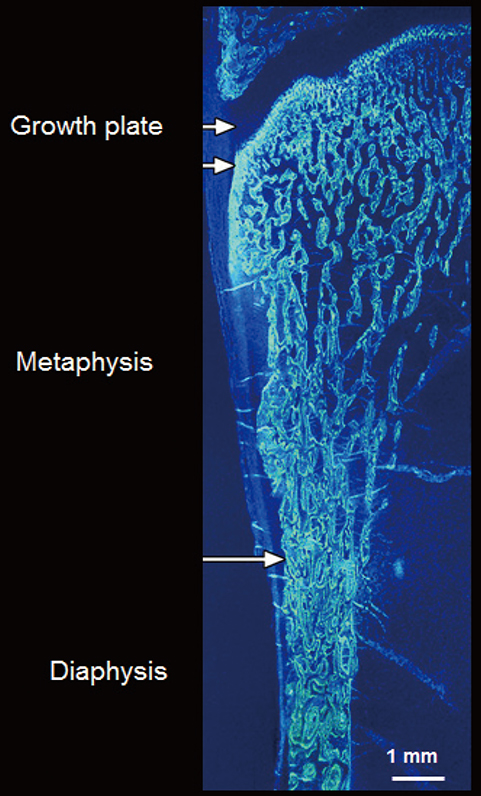
- Vertebral fractures and trabecular bone loss have dominated thinking and research into the pathogenesis and the structural basis of bone fragility during the last 70 years. However, 80% of all fractures are non-vertebral and occur at regions assembled using large amounts of cortical bone; only 20% of fractures are vertebral. Moreover, ~80% of the skeleton is cortical and ~70% of all bone loss is cortical even though trabecular bone is lost more rapidly than cortical bone. Bone is lost because remodelling becomes unbalanced after midlife. Most cortical bone loss occurs by intracortical, not endocortical remodelling. Each remodelling event removes more bone than deposited enlarging existing canals which eventually coalesce eroding and thinning the cortex from 'within.' Thus, there is a need to study the decay of cortical as well as trabecular bone, and to develop drugs that restore the strength of both types of bone. It is now possible to accurately quantify cortical porosity and trabecular decay in vivo. The challenges still to be met are to determine whether measurement of porosity identifies persons at risk for fracture, whether this approach is compliments information obtained using bone densitometry, and whether changes in cortical porosity and other microstructural traits have the sensitivity to serve as surrogates of treatment success or failure.
MeSH Terms
Figure
Reference
-
1. Albright F. Osteoporosis. Ann Intern Med. 1947; 27:861–882.2. Eastell R, Mosekilde L, Hodgson SF, Riggs BL. Proportion of human vertebral body bone that is cancellous. J Bone Miner Res. 1990; 5:1237–1241.3. Rockoff SD, Sweet E, Bleustein J. The relative contribution of trabecular and cortical bone to the strength of human lumbar vertebrae. Calcif Tissue Res. 1969; 3:163–175.4. Hui SL, Slemenda CW, Johnston CC, Appledorn CR. Effects of age and menopause on vertebral bone density. Bone Miner. 1987; 2:141–146.5. Hesp R, Arlot ME, Edouard C, Bradbeer JN, Meunier PJ, Reeve J. Iliac trabecular bone formation predicts radial trabecular bone density changes in type 1 osteoporosis. J Bone Miner Res. 1991; 6:929–935.6. Delmas PD, Fontanges E, Duboeuf F, Boivin G, Chavassieux P, Meunier PJ. Comparison of bone mass measured by histomorphometry on iliac biopsy and by dual photon absorptiometry of the lumbar spine. Bone. 1988; 9:209–213.7. Eastell R, Riggs BL, Wahner HW, O'Fallon WM, Amadio PC, Melton LJ 3rd. Colles' fracture and bone density of the ultradistal radius. J Bone Miner Res. 1989; 4:607–613.8. Eastell R, Wahner HW, O'Fallon WM, Amadio PC, Melton LJ 3rd, Riggs BL. Unequal decrease in bone density of lumbar spine and ultradistal radius in Colles' and vertebral fracture syndromes. J Clin Invest. 1989; 83:168–174.9. Wahner HW. Single- and dual-photon absorptiometry in osteoporosis and osteomalacia. Semin Nucl Med. 1987; 17:305–315.10. Melton LJ 3rd, Atkinson EJ, O'Fallon WM, Wahner HW, Riggs BL. Long-term fracture prediction by bone mineral assessed at different skeletal sites. J Bone Miner Res. 1993; 8:1227–1233.11. Parfitt AM. Misconceptions (2): turnover is always higher in cancellous than in cortical bone. Bone. 2002; 30:807–809.12. Parfitt AM, Mathews CH, Villanueva AR, Kleerekoper M, Frame B, Rao DS. Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. Implications for the microanatomic and cellular mechanisms of bone loss. J Clin Invest. 1983; 72:1396–1409.13. Pouilles JM, Tremollieres F, Ribot C. Spine and femur densitometry at the menopause: are both sites necessary in the assessment of the risk of osteoporosis? Calcif Tissue Int. 1993; 52:344–347.14. Seeman E. Structural basis of growth-related gain and age-related loss of bone strength. Rheumatology (Oxford). 2008; 47:Suppl 4. iv2–iv8.15. Parfitt AM, Travers R, Rauch F, Glorieux FH. Structural and cellular changes during bone growth in healthy children. Bone. 2000; 27:487–494.16. Rauch F, Travers R, Glorieux FH. Intracortical remodeling during human bone development: a histomorphometric study. Bone. 2007; 40:274–280.17. Schnitzler CM, Mesquita JM, Pettifor JM. Cortical bone development in black and white South African children: iliac crest histomorphometry. Bone. 2009; 44:603–611.18. Cadet ER, Gafni RI, McCarthy EF, McCray DR, Bacher JD, Barnes KM, et al. Mechanisms responsible for longitudinal growth of the cortex: coalescence of trabecular bone into cortical bone. J Bone Joint Surg Am. 2003; 85-A:1739–1748.19. Enlow DH. A study of the post-natal growth and remodeling of bone. Am J Anat. 1962; 110:79–101.20. Enlow DH. Principles of bone remodeling: an account of post-natal growth and remodeling processes in long bones and the mandible. Springfield: Thomas Books;1963.21. Wang Q, Ghasem-Zadeh A, Wang XF, Iuliano-Burns S, Seeman E. Trabecular bone of growth plate origin influences both trabecular and cortical morphology in adulthood. J Bone Miner Res. 2011; 26:1577–1583.22. Bala Y, Bui QM, Wang XF, Iuliano S, Wang Q, Ghasem-Zadeh A, et al. Trabecular and cortical microstructure and fragility of the distal radius in women. J Bone Miner Res. 2015; 30:621–629.23. Wang Q, Wang XF, Iuliano-Burns S, Ghasem-Zadeh A, Zebaze R, Seeman E. Rapid growth produces transient cortical weakness: a risk factor for metaphyseal fractures during puberty. J Bone Miner Res. 2010; 25:1521–1526.24. Keshawarz NM, Recker RR. Expansion of the medullary cavity at the expense of cortex in postmenopausal osteoporosis. Metab Bone Dis Relat Res. 1984; 5:223–228.25. Zebaze RM, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010; 375:1729–1736.26. Bergstrom U, Bjornstig U, Stenlund H, Jonsson H, Svensson O. Fracture mechanisms and fracture pattern in men and women aged 50 years and older: a study of a 12-year population-based injury register, Umea, Sweden. Osteoporos Int. 2008; 19:1267–1273.27. Hedstrom EM, Svensson O, Bergstrom U, Michno P. Epidemiology of fractures in children and adolescents. Acta Orthop. 2010; 81:148–153.28. Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000; 15:721–739.29. Havill LM, Allen MR, Harris JA, Levine SM, Coan HB, Mahaney MC, et al. Intracortical bone remodeling variation shows strong genetic effects. Calcif Tissue Int. 2013; 93:472–480.30. Havill LM, Allen MR, Bredbenner TL, Burr DB, Nicolella DP, Turner CH, et al. Heritability of lumbar trabecular bone mechanical properties in baboons. Bone. 2010; 46:835–840.31. Bjornerem A, Bui M, Wang X, Ghasem-Zadeh A, Hopper JL, Zebaze R, et al. Genetic and environmental variances of bone microarchitecture and bone remodeling markers: a twin study. J Bone Miner Res. 2015; 30:519–527.32. Mikkola TM, Sipila S, Rantanen T, Sievanen H, Suominen H, Kaprio J, et al. Genetic and environmental influence on structural strength of weight-bearing and non-weight-bearing bone: a twin study. J Bone Miner Res. 2008; 23:492–498.33. Lips P, Courpron P, Meunier PJ. Mean wall thickness of trabecular bone packets in the human iliac crest: changes with age. Calcif Tissue Res. 1978; 26:13–17.34. Vedi S, Compston JE, Webb A, Tighe JR. Histomorphometric analysis of dynamic parameters of trabecular bone formation in the iliac crest of normal British subjects. Metab Bone Dis Relat Res. 1983; 5:69–74.35. Bjornerem A, Ghasem-Zadeh A, Bui M, Wang X, Rantzau C, Nguyen TV, et al. Remodeling markers are associated with larger intracortical surface area but smaller trabecular surface area: a twin study. Bone. 2011; 49:1125–1130.36. Foldes J, Parfitt AM, Shih MS, Rao DS, Kleerekoper M. Structural and geometric changes in iliac bone: relationship to normal aging and osteoporosis. J Bone Miner Res. 1991; 6:759–766.37. Han ZH, Palnitkar S, Rao DS, Nelson D, Parfitt AM. Effects of ethnicity and age or menopause on the remodeling and turnover of iliac bone: implications for mechanisms of bone loss. J Bone Miner Res. 1997; 12:498–508.38. Zebaze R, Ghasem-Zadeh A, Mbala A, Seeman E. A new method of segmentation of compact-appearing, transitional and trabecular compartments and quantification of cortical porosity from high resolution peripheral quantitative computed tomographic images. Bone. 2013; 54:8–20.39. Zebaze R, Seeman E. Cortical bone: a challenging geography. J Bone Miner Res. 2015; 30:24–29.40. Ruff CB, Hayes WC. Sex differences in age-related remodeling of the femur and tibia. J Orthop Res. 1988; 6:886–896.41. Bouxsein ML. Determinants of skeletal fragility. Best Pract Res Clin Rheumatol. 2005; 19:897–911.42. Holzer G, von Skrbensky G, Holzer LA, Pichl W. Hip fractures and the contribution of cortical versus trabecular bone to femoral neck strength. J Bone Miner Res. 2009; 24:468–474.43. Nawathe S, Nguyen BP, Barzanian N, Akhlaghpour H, Bouxsein ML, Keaveny TM. Cortical and trabecular load sharing in the human femoral neck. J Biomech. 2015; 48:816–822.44. Lotz JC, Cheal EJ, Hayes WC. Stress distributions within the proximal femur during gait and falls: implications for osteoporotic fracture. Osteoporos Int. 1995; 5:252–261.45. Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res. 2008; 23:392–399.46. Schaffler MB, Burr DB. Stiffness of compact bone: effects of porosity and density. J Biomech. 1988; 21:13–16.47. Rice JC, Cowin SC, Bowman JA. On the dependence of the elasticity and strength of cancellous bone on apparent density. J Biomech. 1988; 21:155–168.48. Martin RB, Ishida J. The relative effects of collagen fiber orientation, porosity, density, and mineralization on bone strength. J Biomech. 1989; 22:419–426.49. Burr D. Microdamage and bone strength. Osteoporos Int. 2003; 14:Suppl 5. S67–S72.50. Diab T, Vashishth D. Effects of damage morphology on cortical bone fragility. Bone. 2005; 37:96–102.51. Martin RB, Burr DB. The microscopic structure of bone. New York: Raven Press;1989.52. Yeni YN, Brown CU, Wang Z, Norman TL. The influence of bone morphology on fracture toughness of the human femur and tibia. Bone. 1997; 21:453–459.53. Granke M, Grimal Q, Saied A, Nauleau P, Peyrin F, Laugier P. Change in porosity is the major determinant of the variation of cortical bone elasticity at the millimeter scale in aged women. Bone. 2011; 49:1020–1026.54. Bala Y, Zebaze R, Ghasem-Zadeh A, Atkinson EJ, Iuliano S, Peterson JM, et al. Cortical porosity identifies women with osteopenia at increased risk for forearm fractures. J Bone Miner Res. 2014; 29:1356–1362.55. Siris ES, Chen YT, Abbott TA, Barrett-Connor E, Miller PD, Wehren LE, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004; 164:1108–1112.56. Sanders KM, Nicholson GC, Watts JJ, Pasco JA, Henry MJ, Kotowicz MA, et al. Half the burden of fragility fractures in the community occur in women without osteoporosis. When is fracture prevention cost-effective? Bone. 2006; 38:694–700.57. Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, Seeman E, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004; 34:195–202.58. Ahmed LA, Shigdel R, Joakimsen RM, Eldevik OP, Eriksen EF, Ghasem-Zadeh A, et al. Measurement of cortical porosity of the proximal femur improves identification of women with nonvertebral fragility fractures. Osteoporos Int. 2015; 26:2137–2146.59. Chappard C, Bensalah S, Olivier C, Gouttenoire PJ, Marchadier A, Benhamou C, et al. 3D characterization of pores in the cortical bone of human femur in the elderly at different locations as determined by synchrotron micro-computed tomography images. Osteoporos Int. 2013; 24:1023–1033.60. Biltz RM, Pellegrino ED. The chemical anatomy of bone. I. A comparative study of bone composition in sixteen vertebrates. J Bone Joint Surg Am. 1969; 51:456–466.61. Mueller KH, Trias A, Ray RD. Bone density and compostiton. Age-related and pathological changes in water and mineral content. J Bone Joint Surg Am. 1966; 48:140–148.62. Smith JW. Observations on the water Content of bone. J Bone Joint Surg Br. 1964; 46:553–562.63. Techawiboonwong A, Song HK, Leonard MB, Wehrli FW. Cortical bone water: in vivo quantification with ultrashort echo-time MR imaging. Radiology. 2008; 248:824–833.64. Tjong W, Kazakia GJ, Burghardt AJ, Majumdar S. The effect of voxel size on high-resolution peripheral computed tomography measurements of trabecular and cortical bone microstructure. Med Phys. 2012; 39:1893–1903.65. Geusens P, Chapurlat R, Schett G, Ghasem-Zadeh A, Seeman E, de Jong J, et al. High-resolution in vivo imaging of bone and joints: a window to microarchitecture. Nat Rev Rheumatol. 2014; 10:304–313.66. Nishiyama KK, Macdonald HM, Buie HR, Hanley DA, Boyd SK. Postmenopausal women with osteopenia have higher cortical porosity and thinner cortices at the distal radius and tibia than women with normal aBMD: an in vivo HR-pQCT study. J Bone Miner Res. 2010; 25:882–890.67. Nishiyama KK, Macdonald HM, Moore SA, Fung T, Boyd SK, McKay HA. Cortical porosity is higher in boys compared with girls at the distal radius and distal tibia during pubertal growth: an HR-pQCT study. J Bone Miner Res. 2012; 27:273–282.68. Jordan GR, Loveridge N, Bell KL, Power J, Rushton N, Reeve J. Spatial clustering of remodeling osteons in the femoral neck cortex: a cause of weakness in hip fracture? Bone. 2000; 26:305–313.69. Bell KL, Loveridge N, Reeve J, Thomas CD, Feik SA, Clement JG. Super-osteons (remodeling clusters) in the cortex of the femoral shaft: influence of age and gender. Anat Rec. 2001; 264:378–386.70. Pfeiffer S, Crowder C, Harrington L, Brown M. Secondary osteon and Haversian canal dimensions as behavioral indicators. Am J Phys Anthropol. 2006; 131:460–468.71. Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005; 90:6508–6515.72. Stein EM, Liu XS, Nickolas TL, Cohen A, Thomas V, McMahon DJ, et al. Abnormal microarchitecture and reduced stiffness at the radius and tibia in postmenopausal women with fractures. J Bone Miner Res. 2010; 25:2572–2581.73. Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res. 2007; 22:425–433.74. Bala Y, Chapurlat R, Cheung AM, Felsenberg D, LaRoche M, Morris E, et al. Risedronate slows or partly reverses cortical and trabecular microarchitectural deterioration in postmenopausal women. J Bone Miner Res. 2014; 29:380–388.75. Burghardt AJ, Kazakia GJ, Sode M, de Papp AE, Link TM, Majumdar S. A longitudinal HR-pQCT study of alendronate treatment in postmenopausal women with low bone density: relations among density, cortical and trabecular microarchitecture, biomechanics, and bone turnover. J Bone Miner Res. 2010; 25:2558–2571.76. Zebaze RM, Libanati C, Austin M, Ghasem-Zadeh A, Hanley DA, Zanchetta JR, et al. Differing effects of denosumab and alendronate on cortical and trabecular bone. Bone. 2014; 59:173–179.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Clinical Study of the Children's Ankle Fractures with Growth Plate Injury
- Cortical Perforation Misidentified with Medial Condylar Fracture of Femur in Total Knee Arthroplasty: Case Report
- Follow - up Evaluation after Saucerization of the Chronic Osteomyelitis of Long Bones
- Proximal Humeral Fracture with Epiphyseal Plate Injury
- Risk Factors in Progression of Deformity in Compression Fracture of Thoracolumbar Junction