J Korean Rheum Assoc.
2007 Mar;14(1):23-30. 10.4078/jkra.2007.14.1.23.
The Effect of Estrogen on the DNA Methylation of B Cells in Patients with SLE
- Affiliations
-
- 1The Rheumatism Research Center (RhRC), Catholic Research Institute of Medical Science, The Catholic University of Korea, Seoul, Korea. rapark@catholic.ac.kr
- KMID: 2202187
- DOI: http://doi.org/10.4078/jkra.2007.14.1.23
Abstract
OBJECTIVE
Epigenetics is an important, alternative mechanism of gene regulation that is independent of the nucleotide sequences of DNA. We investigated mRNA levels for DNA methyltransferase-1 (DNMT-1), and the effect of estrogen on the expression of DNMT-1 level in T cells and B cells from patients with systemic lupus erythematosus (SLE) and healthy subjects, and assessed the possible etiological role of DNA methylation in the pathogenesis of SLE.
METHODS
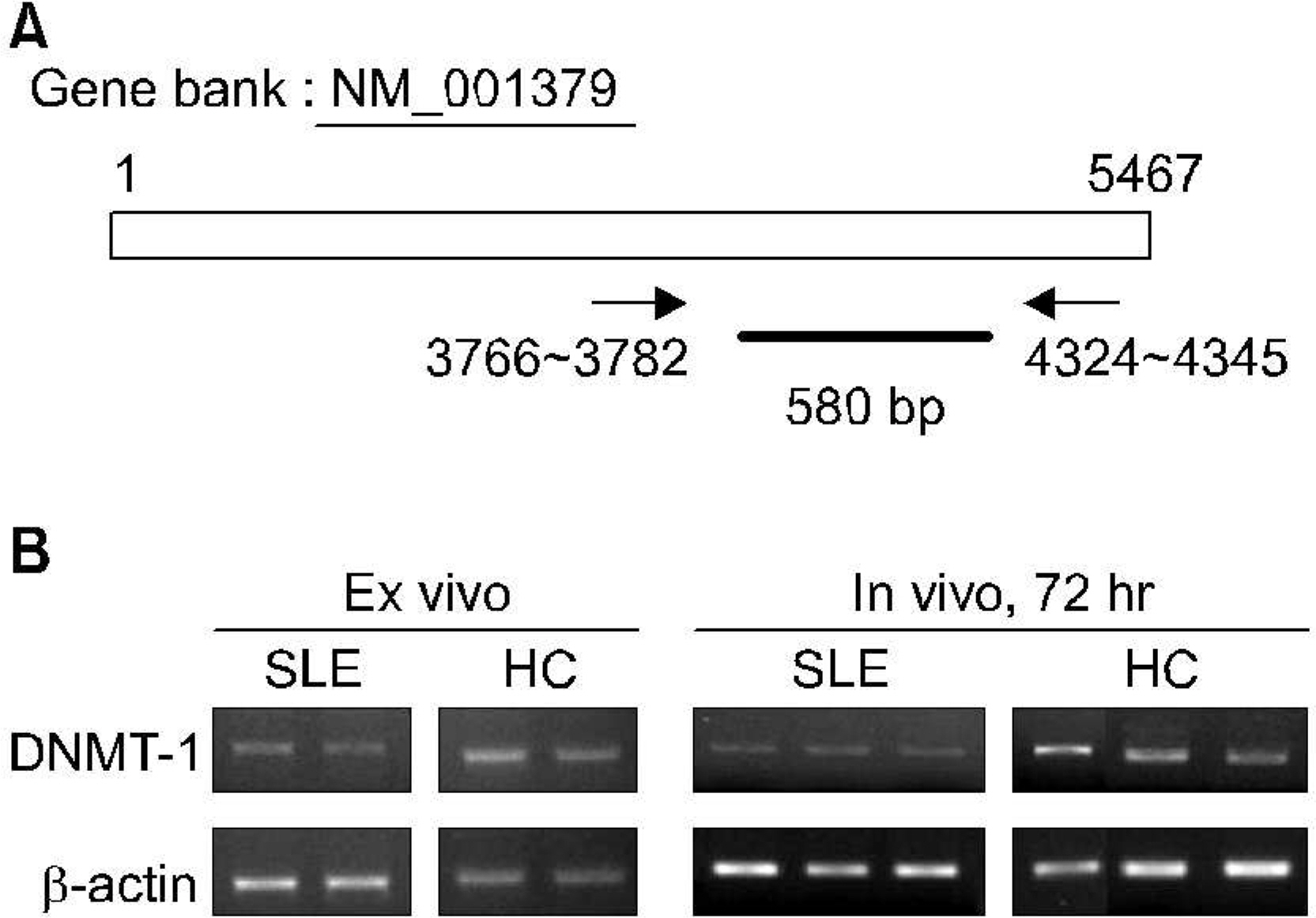
mRNA levels for DNMT-1 in CD4+ T cells and CD19+ B cells from 37 patients with SLE and 12 healthy controls were examined using RT-PCR. We used specific primer for DNMT-1 and beta actin, The effect of estrogen on the DNA methylation was measured by the mRNA level of DNMT-1 CD4+ T cells and CD19+ B cells treated with 100 nM of 17beta-estradiol for 72 hour.
RESULTS
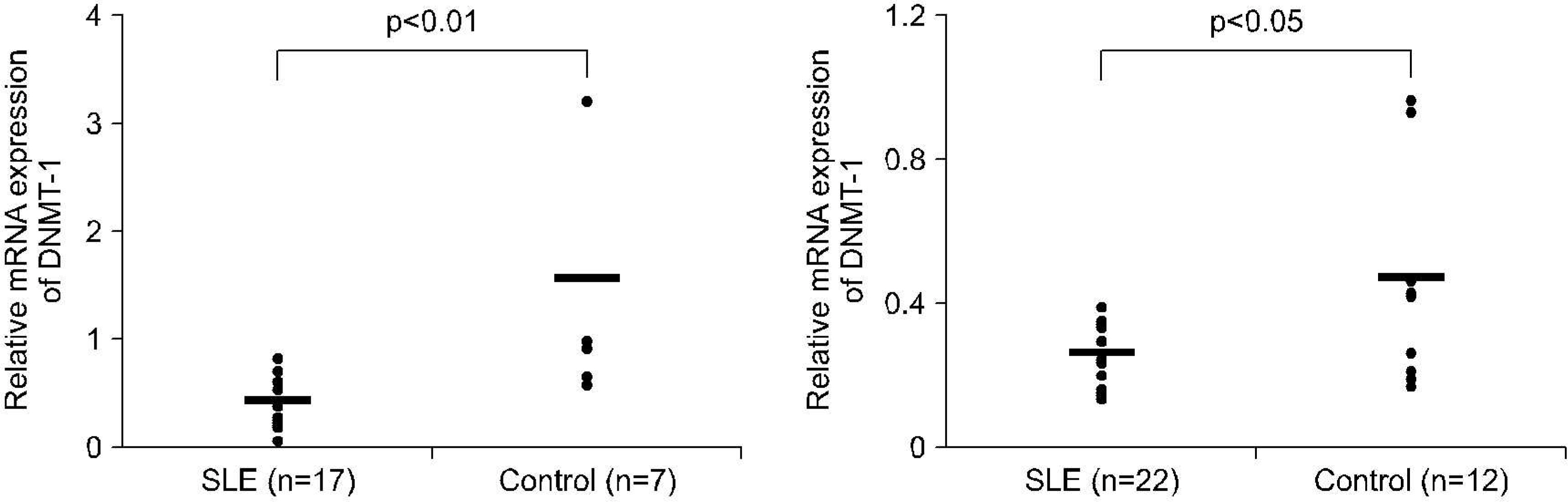
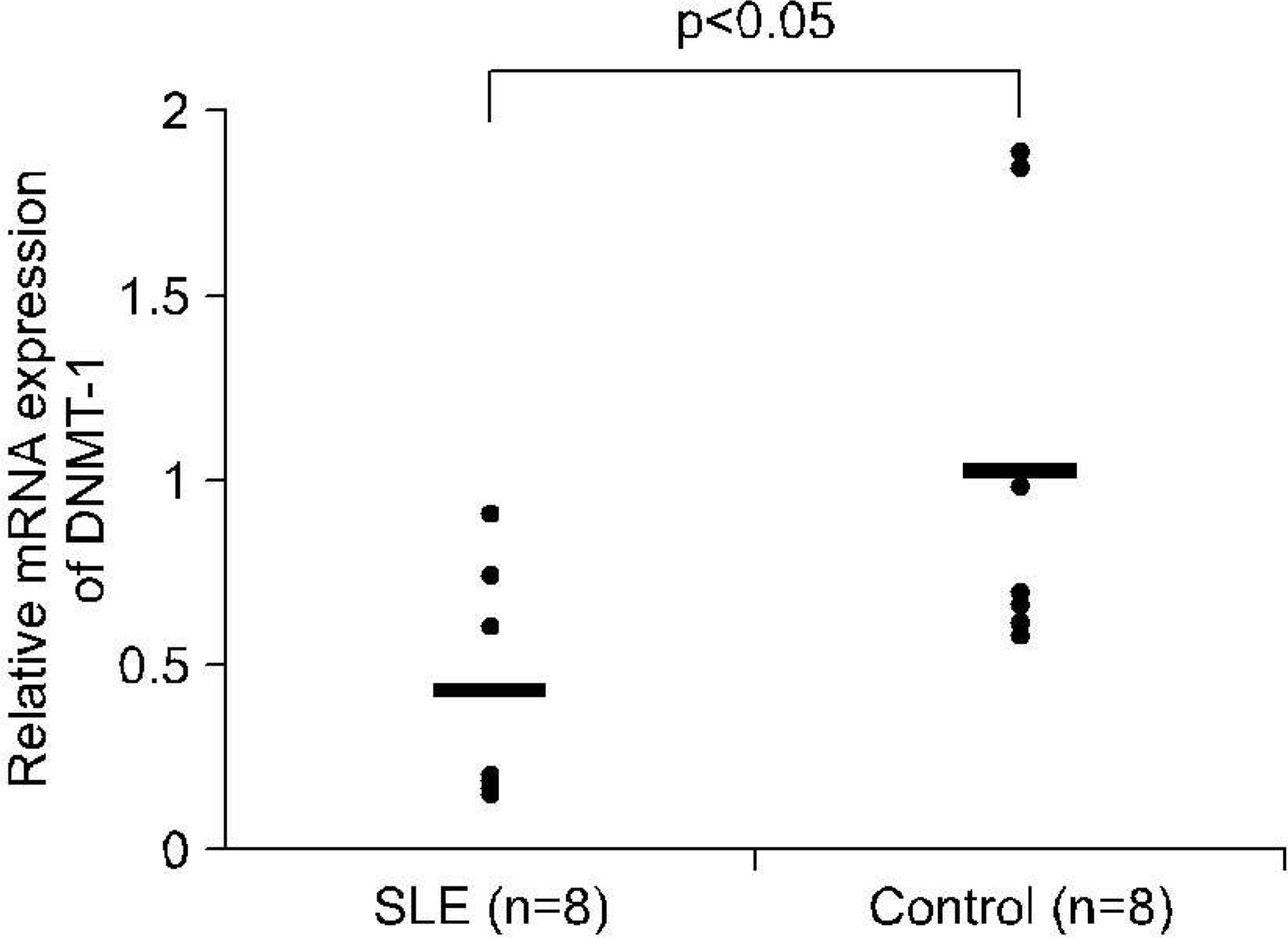
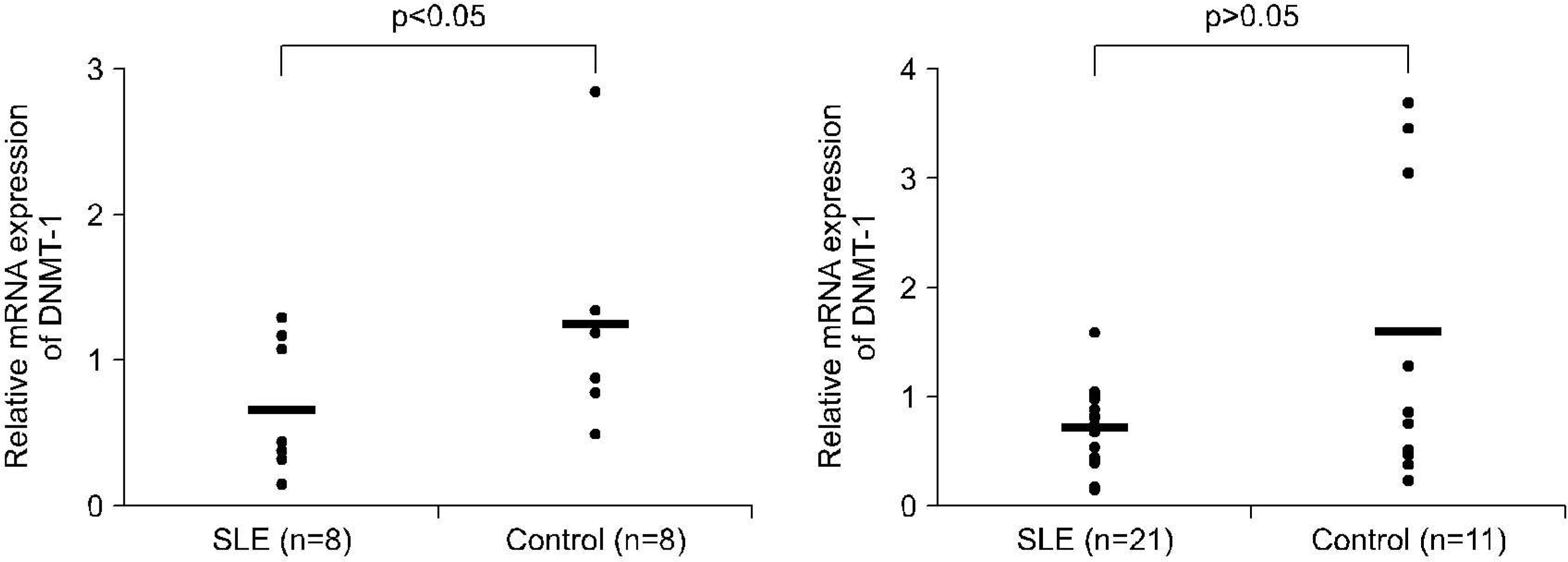
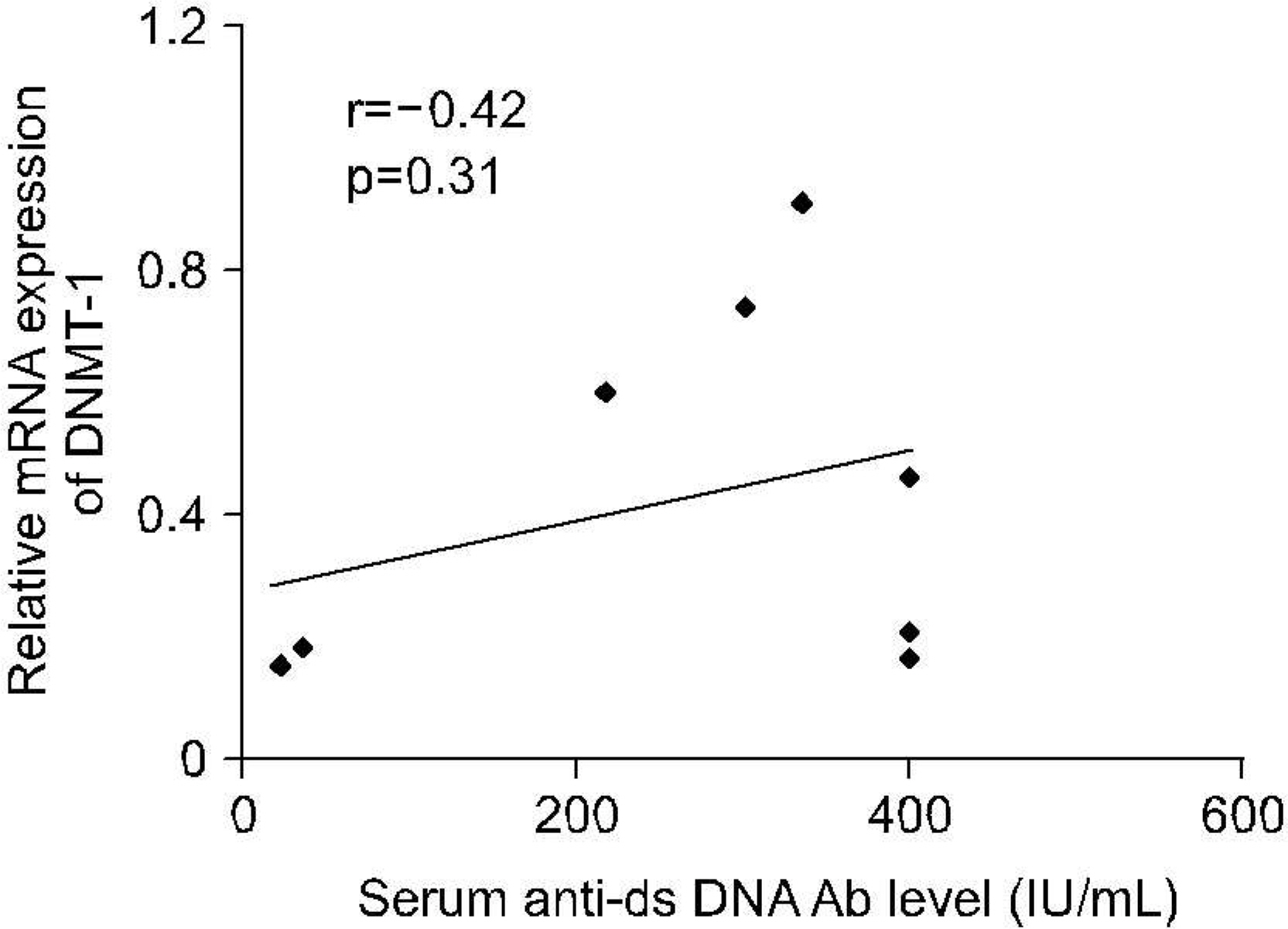
The levels of DNMT-1 mRNA were significantly lower in CD4+ T cells and CD19+ B cells from SLE patients compared with healthy controls. We observed the suppression of the levels of DNMT-1 mRNA by stimulated with estrogen in patients with SLE patients, especially in CD19+B cells. DNA hypomethylation of B cells was tend to be correlated with the level of anti-ds DNA antibody without statistical significance (r=-0.43, p=0.3).
CONCLUSION
Our observations suggest that suppression of DNMT-1 by estrogen in B cells from patients with SLE might be related to the pathogenesis of SLE. Epigenetic studies may provide clues for developing new treatment strategies of SLE.
MeSH Terms
Figure
Reference
-
1). Mills JA. Systemic lupus erythematosus. N Eng J Med. 1994. 330:1871–9.
Article2). Boumpas DT., Austin HA 3rd., Fessler BJ., Balow JE., Klippel JH., Lockshin MD. Systemic lupus erythematosus: emerging concepts. Part 1: renal, neuropsychiatric, cardiovascular, pulmonary, and hematologic disease. Ann Intern Med. 1995. 122:940–50.
Article3). Boumpas DT., Fessler BJ., Austin HA 3rd., Balow JE., Klippel JH., Lockshin MD. Systemic lupus erythematosus: emerging concepts. Part 2: dermatologic and joint disease, the antiphospholipid antibody syndrome, pregnancy and hormonal therapy, morbidity and mortality, and pathogenesis. Ann Intern Med. 1995. 123:42–53.
Article4). Wolffe AP., Matzke MA. Epigenetics: regulation through repression. Science. 1999. 286:481–6.
Article5). Richardson B., Yung R. Role of DNA methylation in the regulation of cell function. J Lab Clin Med. 1999. 134:333–40.
Article6). Nakao M. Epigenetics: interaction of DNA methylation and chromatin. Gene. 2001. 278:25–31.
Article7). Bestor TH. Activation of mammalian DNA methyl-transferase by cleavage of a Zn binding regulatory domain. EMBO J. 1992. 11:2611–7.
Article8). Okano M., Xie S., Li E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat Genet. 1998. 19:219–20.
Article9). Richardson B., Powers D., Hooper F., Yung RL., O'Rourke K. Lymphocyte function-associated antigen 1 overexpression and T cell autoreactivity. Arthritis Rheum. 1994. 37:1363–72.
Article10). Yung R., Powers D., Johnson K., Amento E., Carr D., Laing T, et al. Richardson B. Mechanisms of drug-induced lupus. II. T cells overexpressing lymphocyte function-associated antigen 1 become autoreactive and cause a lupuslike disease in syngeneic mice. J Clin Invest. 1996. 97:2866–71.11). Kaplan MJ., Deng C., Yang J., Richardson BC. DNA methylation in the regulation of T cell LFA-1 expression. Immunol Invest. 2000. 29:411–25.12). Lu Q., Wu A., Richardson BC. Demethylation of the same promoter sequence increases CD70 expression in lupus T cells and T cells treated with lupus-inducing drugs. J Immunol. 2005. 174:6212–9.
Article13). ᄋelke K., Lu Q., Richardson D., Wu A., Deng C., Hanash S, et al. Overexpression of CD70 and overstimulation of IgG synthesis by lupus T cells and T cells treated with DNA methylation inhibitors. Arthritis Rheum. 2004. 50:1850–60.14). Kaplan MJ., Lu Q., Wu A., Attwood J., Richardson B. Demethylation of promoter regulatory elements contributes to perforin overexpression in CD4 + lupus T cells. J Immunol. 2004. 172:3652–61.15). Masi AT., Kaslow RA. Sex effects in systemic lupus erythematosus: a clue to pathogenesis. Arthritis Rheum. 1978. 21:480–4.
Article16). Sekigawa I., Naito T., Hira K., Mitsuishi K., ᄋgasawara H., Hashimoto H, et al. Possible mechanisms of gender bias in systemic lupus erythematosus: a new hypothesis involving a comparison of systemic lupus erythematosus with atopy. Lupus. 2004. 13:217–22.17). Cutolo M., Sulli A., Seriolo B., Accardo S., Masi AT. Estrogens, the immune response and autoimmunity. Clin Exp Rheumatol. 1995. 13:217–26.18). Cutolo M., Sulli A., Villaggio B., Seriolo B., Accardo S. Relations between steroid hormones and cytokines in rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis. 1998. 57:573–7.
Article19). Verthelyi D. Sex hormones as immunomodulators in health and disease. Int Immunopharmacol. 2001. 1:983–93.
Article20). ᄋkada M., ᄋgasawara H., Kaneko H., Hishikawa T., Sekiawa I., Hashimoto H, et al. Role of DNA methylation in the transcription of human endogenous retroviruses in the pathogenesis of systemic lupus erythematosus. J Rheumatol. 2002. 29:1678–82.21). ᄋgasawara H., ᄋkada M., Kaneko H., Hishikawa T., Sekigawa I., Hashimoto H. Possible role of DNA hypomethylation in the induction of SLE: relationship to the transcription of human endogenous retroviruses. Clin Exp Rheumatol. 2003. 21:733–8.22). Groudine M., Eisenman R., Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981. 292:311–7.
Article23). Sekigawa I., Kawasaki M., ᄋgasawara H., Kaneda K., Kaneko H., Takasaki Y, et al. DNA methylation: its contribution to systemic lupus erythematosus. Clin Exp Med. 2006. 6:99–106.
Article24). Ballestar E. Esteller M, Richardson BC. The epigenetic face of systemic lupus erythematosus J Immunol. 2006. 176:7143–47.25). Grimaldi CM. Sex and systemic lupus erythematosus: the role of the sex hormones estrogen and prolactin on the regulation of autoreactive B cells. Curr ᄋpin Rheumatol. 2006. 18:456–61.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- DNA Methylation in Development
- DNA methylation: an epigenetic mark of cellular memory
- DNA methylation: a cause and consequence of type 2 diabetes
- Role of DNA Methylation in the Development and Differentiation of Intestinal Epithelial Cells and Smooth Muscle Cells
- A Visualization Tool for Computational Analysis of DNA Methylation Level Using Bisulfite Sequencing Data