J Korean Soc Hypertens.
2013 Mar;19(1):23-38. 10.5646/jksh.2013.19.1.23.
Influence of PD 123319 (AT2-Receptor Antagonist) on Catecholamine Secretion in the Perfused Rat Adrenal Medulla
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Chosun University School of Medicine, Gwangju, Korea.
- 2Department of Pharmacology, Chosun University School of Medicine, Gwangju, Korea. dylim@chosun.ac.kr
- 3Division of Sport Physiology, Department of Leasure and Soport, Dongshin University College of Public Health and Welfare, Naju, Korea.
- 4Department of Physical Education, Chosun University Graduate School, Gwangju, Korea.
- KMID: 2196654
- DOI: http://doi.org/10.5646/jksh.2013.19.1.23
Abstract
- BACKGROUND
The aim of this study was to examine whether PD 123319 (an angiotensin II type 2 [AT2] receptor antagonist) can influence the release of catecholamines (CA) from the perfused model of the rat adrenal medulla.
METHODS
The adrenal gland was isolated by the modification of Wakade method, and perfused with normal Krebs-bicarbonate solution. The content of CA was measured using the fluorospectrophotometer.
RESULTS
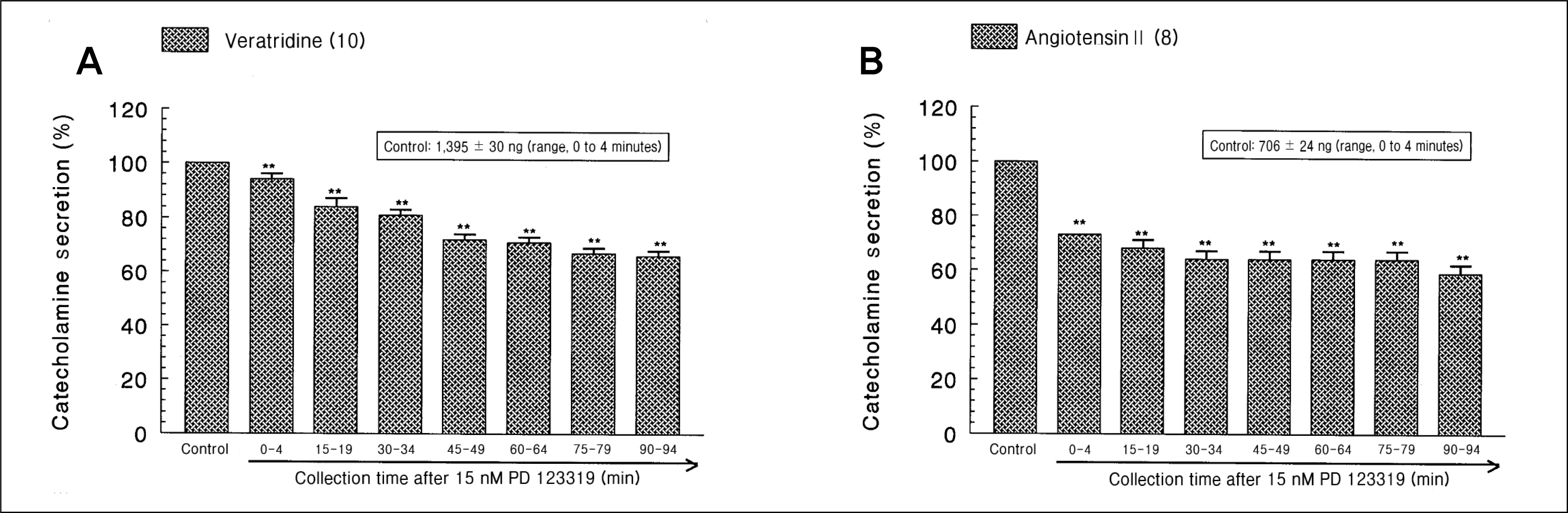
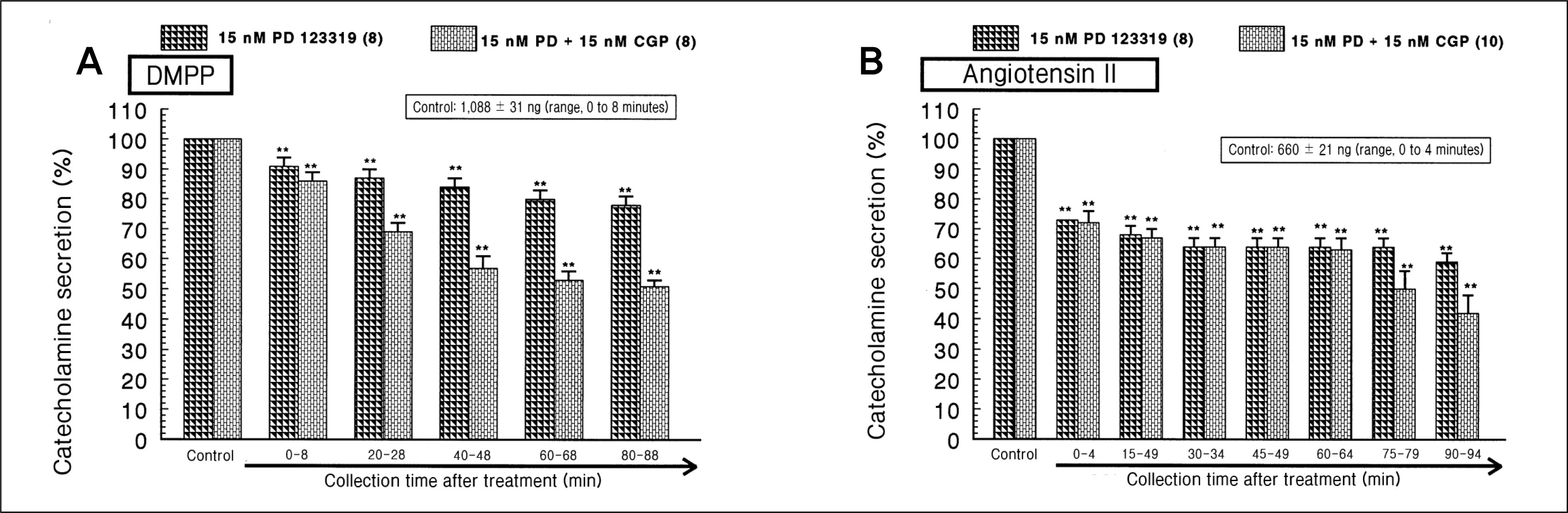
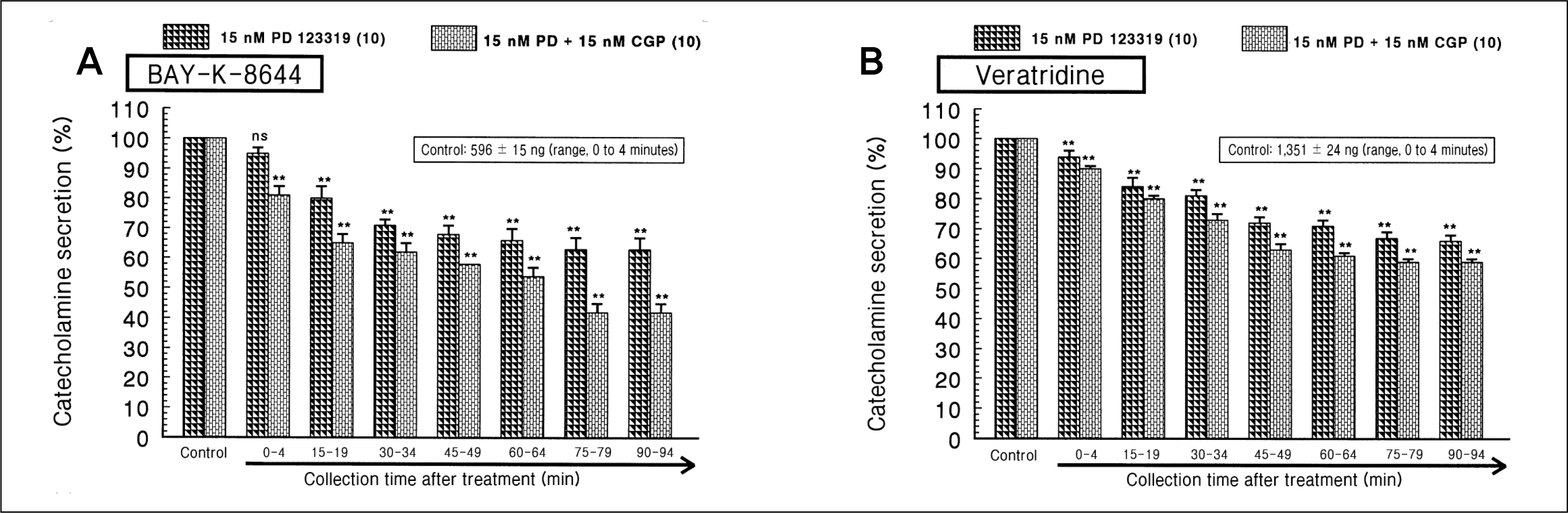
During perfusion of PD 123319 (range, 5 to 50 nM) into an adrenal vein for 90 minutes the CA secretory responses evoked by acetylcholine (ACh), high K+, 1,1-dimethyl-4-phenylpiperazinium iodide (DMPP), and McN-A-343 was dose- and time-dependently inhibited. Furthermore, loading with PD 123319 for 90 minutes also markedly inhibited the CA secretory responses evoked by 4-dihydro-2,6-dimethyl-3-nitro-4-(2-trifluoro-methyl-phenyl)-pyridine-5-carboxylate (Bay-K-8644), cyclopiazonic acid, veratridine, and angiotensin II (Ang II). PD 123319 did not affect basal CA output. Simultaneous perfusion of PD 123319 and CGP 42112 perfused into an adrenal vein for 90 minutes rather more potently inhibited the CA seretory responses evoked by Ach, high K+, DMPP, Bay-K-8644, veratridine, and Ang II compared to the inhibitory effect by PD123319-treated alone.
CONCLUSIONS
Taken together, these results show that PD 123319 inhibits the CA secretion evoked by both cholinergic and Ang II receptor stimulation from the perfused rat adrenal medulla. This inhibitory effect of PD 123319 seems to be exerted by blocking the influx of both Na+ and Ca2+ through their voltage-dependent channels into the rat adrenomedullary chromaffin cells as well as by reducing the Ca2+ release from its cytoplasmic calcium store, which may be relevant to AT2 receptor blockade. Based on these present data, it is thought that PD 123319 has different activity from previously known AT2 antagonist activity in the perfused adrenal medulla, and that AT2 receptors may be involved in the rat adrenomedullary CA secretion.
Keyword
MeSH Terms
-
(4-(m-Chlorophenylcarbamoyloxy)-2-butynyl)trimethylammonium Chloride
3-Pyridinecarboxylic acid, 1,4-dihydro-2,6-dimethyl-5-nitro-4-(2-(trifluoromethyl)phenyl)-, Methyl ester
Acetylcholine
Adrenal Glands
Adrenal Medulla
Angiotensin II
Angiotensin II Type 2 Receptor Blockers
Animals
Calcium
Catecholamines
Chromaffin Cells
Cytoplasm
Dimethylphenylpiperazinium Iodide
Imidazoles
Indoles
Oligopeptides
Perfusion
Pyridines
Rats
Veins
Veratridine
(4-(m-Chlorophenylcarbamoyloxy)-2-butynyl)trimethylammonium Chloride
3-Pyridinecarboxylic acid, 1,4-dihydro-2,6-dimethyl-5-nitro-4-(2-(trifluoromethyl)phenyl)-, Methyl ester
Acetylcholine
Angiotensin II
Angiotensin II Type 2 Receptor Blockers
Calcium
Catecholamines
Dimethylphenylpiperazinium Iodide
Imidazoles
Indoles
Oligopeptides
Pyridines
Veratridine
Figure
Reference
-
References
1. Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, et al. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993; 45:205–51.2. Mukoyama M, Nakajima M, Horiuchi M, Sasamura H, Pratt RE, Dzau VJ. Expression cloning of type 2 angiotensin II receptor reveals a unique class of seven-transmembrane receptors. J Biol Chem. 1993; 268:24539–42.
Article3. Kambayashi Y, Bardhan S, Takahashi K, Tsuzuki S, Inui H, Hamakubo T, et al. Molecular cloning of a novel angiotensin II receptor isoform involved in phosphotyrosine phosphatase inhibition. J Biol Chem. 1993; 268:24543–6.
Article4. Unger T, Chung O, Csikos T, Culman J, Gallinat S, Gohlke P, et al. Angiotensin receptors. J Hypertens Suppl. 1996; 14:S95–103.5. Ardaillou R. Angiotensin II receptors. J Am Soc Nephrol. 1999; 10(Suppl 11):S30–9.6. Wong PC, Hart SD, Zaspel AM, Chiu AT, Ardecky RJ, Smith RD, et al. Functional studies of nonpeptide angiotensin II receptor subtype-specific ligands: DuP 753 (AII-1) and PD123177 (AII-2). J Pharmacol Exp Ther. 1990; 255:584–92.7. Dendorfer A, Raasch W, Tempel K, Dominiak P. Interactions between the renin-angiotensin system (RAS) and the sympathetic system. Basic Res Cardiol. 1998; (93):(Suppl 2):24–9.
Article8. Stoehr SJ, Smolen JE, Holz RW, Agranoff BW. Inositol trisphosphate mobilizes intracellular calcium in permeabilized adrenal chromaffin cells. J Neurochem. 1986; 46:637–40.
Article9. Dunn LA, Holz RW. Catecholamine secretion from digitonin-treated adrenal medullary chromaffin cells. J Biol Chem. 1983; 258:4989–93.
Article10. Balla T, Baukal AJ, Eng S, Catt KJ. Angiotensin II receptor subtypes and biological responses in the adrenal cortex and medulla. Mol Pharmacol. 1991; 40:401–6.11. Hano T, Mizukoshi M, Baba A, Nakamura N, Nishio I. Angiotensin II subtype 1 receptor modulates epinephrine release from isolated rat adrenal gland. Blood Press Suppl. 1994; 5:105–8.12. Martineau D, Yamaguchi N, Briand R. Inhibition by BMS 186295, a selective nonpeptide AT1 antagonist, of adrenal catecholamine release induced by angiotensin II in the dog in vivo. Can J Physiol Pharmacol. 1995; 73:459–64.
Article13. Bunn SJ, Marley PD. Effects of angiotensin II on cultured, bovine adrenal medullary cells. Neuropeptides. 1989; 13:121–32.
Article14. Powis DA. O’Brien KJ. Angiotensin II increases catecholamine release from bovine adrenal medulla but does not enhance that evoked by K+ depolarization or by carbachol. J Neurochem. 1991; 57:1461–9.15. Israel A, Stromberg C, Tsutsumi K, Garrido MR, Torres M, Saavedra JM. Angiotensin II receptor subtypes and phosphoinositide hydrolysis in rat adrenal medulla. Brain Res Bull. 1995; 38:441–6.
Article16. Lu X, Grove KL, Zhang W, Speth RC. Pharmacological characterization of angiotensin II AT(2) receptor subtype heterogeneity in the rat adrenal cortex and medulla. Endocrine. 1995; 3:255–61.
Article17. Chiu AT, Herblin WF, McCall DE, Ardecky RJ, Carini DJ, Duncia JV, et al. Identification of angiotensin II receptor subtypes. Biochem Biophys Res Commun. 1989; 165:196–203.
Article18. Whitebread S, Mele M, Kamber B. de Gasparo M. Preliminary biochemical characterization of two angiotensin II receptor subtypes. Biochem Biophys Res Commun. 1989; 163:284–91.19. Belloni AS, Andreis PG, Macchi V, Gottardo G, Malendowicz LK, Nussdorfer GG. Distribution and functional significance of angiotensin-II AT1- and AT2-receptor subtypes in the rat adrenal gland. Endocr Res. 1998; 24:1–15.20. Mazzocchi G, Gottardo G, Macchi V, Malendowicz LK, Nussdorfer GG. The AT2 receptor-mediated stimulation of adrenal catecholamine release may potentiate the AT1 receptor-mediated aldosterone secretagogue action of angiotensin-II in rats. Endocr Res. 1998; 24:17–28.21. Takekoshi K, Ishii K, Kawakami Y, Isobe K, Nakai T. Activation of angiotensin II subtype 2 receptor induces catecholamine release in an extracellular Ca(2+)-dependent manner through a decrease of cyclic guanosine 3’,5’-monophosphate production in cultured porcine adrenal medullary chromaffin Cells. Endocrinology. 2001; 142:3075–86.
Article22. Takekoshi K, Ishii K, Isobe K, Nanmoku T, Kawakami Y, Nakai T. Angiotensin-II subtype 2 receptor agonist (CGP-42112) inhibits catecholamine biosynthesis in cultured porcine adrenal medullary chromaffin cells. Biochem Biophys Res Commun. 2000; 272:544–50.
Article23. Martineau D, Lamouche S, Briand R, Yamaguchi N. Functional involvement of angiotensin AT2 receptor in adrenal catecholamine secretion in vivo. Can J Physiol Pharmacol. 1999; 77:367–74.24. Nahmias C, Strosberg AD. The angiotensin AT2 receptor: searching for signal-transduction pathways and physiological function. Trends Pharmacol Sci. 1995; 16:223–5.
Article25. Wakade AR. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J Physiol. 1981; 313:463–80.
Article26. Anton AH, Sayre DF. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962; 138:360–75.27. Tallarida RJ, Murray RB. Manual of pharmacologic calculations with computer programs. 2nd ed. New York: Springer-Verlag. 1987; 132.28. Ishikawa K, Kanno T. Influences of extracellular calcium and potassium concentrations on adrenaline release and membrane potential in the perfused adrenal medulla of the rat. Jpn J Physiol. 1978; 28:275–89.
Article29. Ohta T, Wakade AR, Yonekubo K, Ito S. Functional relation between caffeine- and muscarine-sensitive Ca2+ stores and no Ca2+ releasing action of cyclic adenosine diphosphate-ribose in guinea-pig adrenal chromaffin cells. Neurosci Lett. 2002; 326:167–70.
Article30. Hammer R, Giachetti A. Muscarinic receptor subtypes: M1 and M2 biochemical and functional characterization. Life Sci. 1982; 31:2991–8.
Article31. Garcia AG, Sala F, Reig JA, Viniegra S, Frias J, Fonteriz R, et al. Dihydropyridine BAY-K-8644 activates chromaffin cell calcium channels. Nature. 1984; 309:69–71.
Article32. Lim DY, Kim CD, Ahn GW. Influence of TMB-8 on secretion of catecholamines from the perfused rat adrenal glands. Arch Pharm Res. 1992; 15:115–25.
Article33. Goeger DE, Riley RT. Interaction of cyclopiazonic acid with rat skeletal muscle sarcoplasmic reticulum vesicles. Effect on Ca2+ binding and Ca2+ permeability. Biochem Pharmacol. 1989; 38:3995–4003.34. Seidler NW, Jona I, Vegh M, Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989; 264:17816–23.
Article35. Wada A, Takara H, Izumi F, Kobayashi H, Yanagihara N. Influx of 22Na through acetylcholine receptor-associated Na channels: relationship between 22Na influx, 45Ca influx and secretion of catecholamines in cultured bovine adrenal medulla cells. Neuroscience. 1985; 15:283–92.
Article36. Bumpus FM, Catt KJ, Chiu AT, DeGasparo M, Goodfriend T, Husain A, et al. Nomenclature for angiotensin receptors: a report of the Nomenclature Committee of the Council for High Blood Pressure Research. Hypertension. 1991; 17:720–1.
Article37. De Gasparo M, Catt KJ, Inagami T. Angiotensin receptors. Girdlestone D, editor. The IUPHAR compendium receptor characterization and classification. Cambridge: The Burlington Press;1998. p. 80–6.38. Pelet C, Mironneau C, Rakotoarisoa L, Neuilly G. Angiotensin II receptor subtypes and contractile responses in portal vein smooth muscle. Eur J Pharmacol. 1995; 279:15–24.
Article39. Defaye G, Lecomte S, Chambaz EM, Bottari SP. Stimulation of cortisol production through angiotensin AT2 receptors in bovine fasciculata cells. Endocr Res. 1995; 21:183–7.40. Whitebread SE, Taylor V, Bottari SP, Kamber B. de Gasparo M. Radioiodinated CGP 42112A: a novel high affinity and highly selective ligand for the characterization of angiotensin AT2 receptors. Biochem Biophys Res Commun. 1991; 181:1365–71.41. Heemskerk FM, Saavedra JM. Quantitative autoradiography of angiotensin II AT2 receptors with [125I]CGP 42112. Brain Res. 1995; 677:29–38.
Article42. Brechler V, Jones PW, Levens NR, de Gasparo M, Bottari SP. Agonistic and antagonistic properties of angiotensin analogs at the AT2 receptor in PC12W cells. Regul Pept. 1993; 44:207–13.
Article43. Lokuta AJ, Cooper C, Gaa ST, Wang HE, Rogers TB. Angiotensin II stimulates the release of phospholipid-derived second messengers through multiple receptor subtypes in heart cells. J Biol Chem. 1994; 269:4832–8.
Article44. Schafer F, Muller AR, Schmid HA, Gerstberger R, Simon E. Angiotensin II receptor subtypes in the duck subfornical organ: an electrophysiological and receptor autoradiographic investigation. Brain Res. 1996; 711(1-2):118–24.45. Sasaoka T, Egi Y, Tawa M, Yamamoto A, Ohkita M, Takaoka M, et al. Angiotensin II type 2 receptor-mediated inhibition of norepinephrine release in isolated rat hearts. J Cardiovasc Pharmacol. 2008; 52:176–83.
Article46. Noh HJ, Kang YS, Lim DY. Effects of losartan on catecholamine release in the isolated rat adrenal gland. Korean J Physiol Pharmacol. 2009; 13:327–35.
Article47. Worck RH, Frandsen E, Ibsen H, Petersen JS. AT1 and AT2 receptor blockade and epinephrine release during insulin-induced hypoglycemia. Hypertension. 1998; 31(1 Pt 2):384–90.48. Armando I, Jezova M, Bregonzio C, Baiardi G, Saavedra JM. Angiotensin II AT1 and AT2 receptor types regulate basal and stress-induced adrenomedullary catecholamine production through transcriptional regulation of tyrosine hydroxylase. Ann N Y Acad Sci. 2004; 1018:302–9.49. McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol. 1995; 57:521–46.
Article50. Cheek TR, O’Sullivan AJ, Moreton RB, Berridge MJ, Burgoyne RD. Spatial localization of the stimulus-induced rise in cytosolic Ca2+ in bovine adrenal chromaffin cells: distinct nicotinic and muscarinic patterns. FEBS Lett. 1989; 247:429–34.51. Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995; 268:239–47.
Article52. Holz RW, Senter RA, Frye RA. Relationship between Ca2+ uptake and catecholamine secretion in primary dissociated cultures of adrenal medulla. J Neurochem. 1982; 39:635–46.53. Suzuki M, Muraki K, Imaizumi Y, Watanabe M. Cyclopiazonic acid, an inhibitor of the sarcoplasmic reticulum Ca(2+)-pump, reduces Ca(2+)-dependent K+ currents in guinea-pig smooth muscle cells. Br J Pharmacol. 1992; 107:134–40.
Article54. Challis RA, Jones JA, Owen PJ, Boarder MR. Changes in inositol 1,4,5-trisphosphate and inositol 1,3,4,5- tetraki -sphosphate mass accumulations in cultured adrenal chromaffin cells in response to bradykinin and histamine. J Neurochem. 1991; 56:1083–6.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gintonin facilitates catecholamine secretion from the perfused adrenal medulla
- Influence of SKF81297 on Catecholamine Release from the Perfused Rat Adrenal Medulla
- Inhibitory Effects of Olmesartan on Catecholamine Secretion from the Perfused Rat Adrenal Medulla
- D-Amphetamine Causes Dual Actions on Catecholamine Release from the Rat Adrenal Medulla
- Influence of Bradykinin on Catecholamine Release from the Rat Adrenal Medulla