Korean J Physiol Pharmacol.
2016 Nov;20(6):629-639. 10.4196/kjpp.2016.20.6.629.
Gintonin facilitates catecholamine secretion from the perfused adrenal medulla
- Affiliations
-
- 1Department of Physiology, College of Veterinary Medicine, Konkuk University, Seoul 05029, Korea.
- 2Department of Internal Medicine, School of Medicine, Boramae Seoul National University, Seoul 07061, Korea.
- 3Department of Leisure & Sport, College of Public Health & Welfare, Dongshin University, Naju 58245, Korea.
- 4Department of Psychiatry, College of Medicine, Chosun University, Gwangju 61452, Korea.
- 5Department of Pharmacology, College of Medicine, Chosun University, Gwangju 61452, Korea. dylim@chosun.ac.kr
- KMID: 2364267
- DOI: http://doi.org/10.4196/kjpp.2016.20.6.629
Abstract
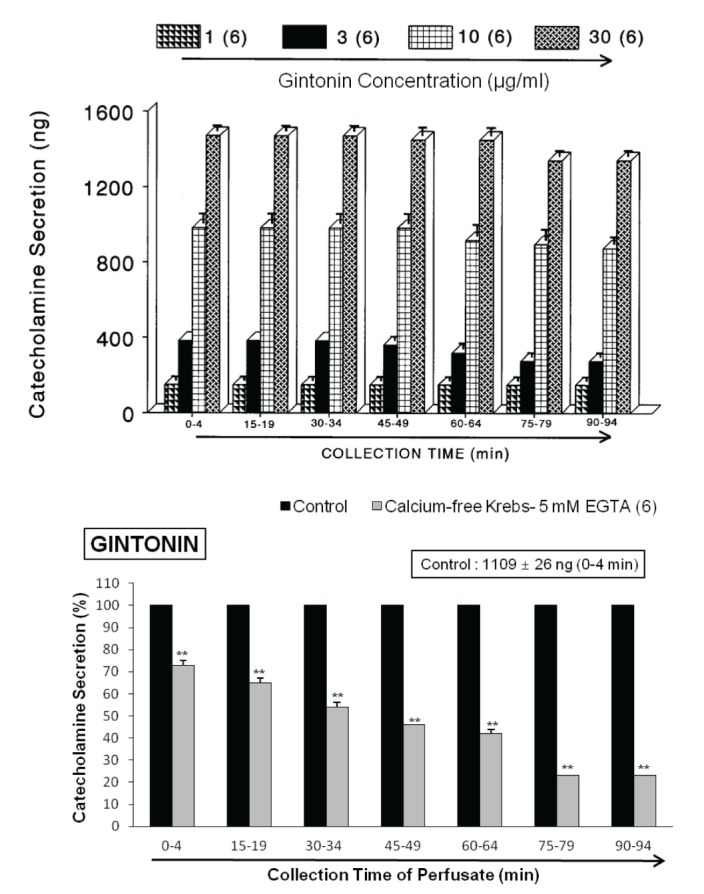
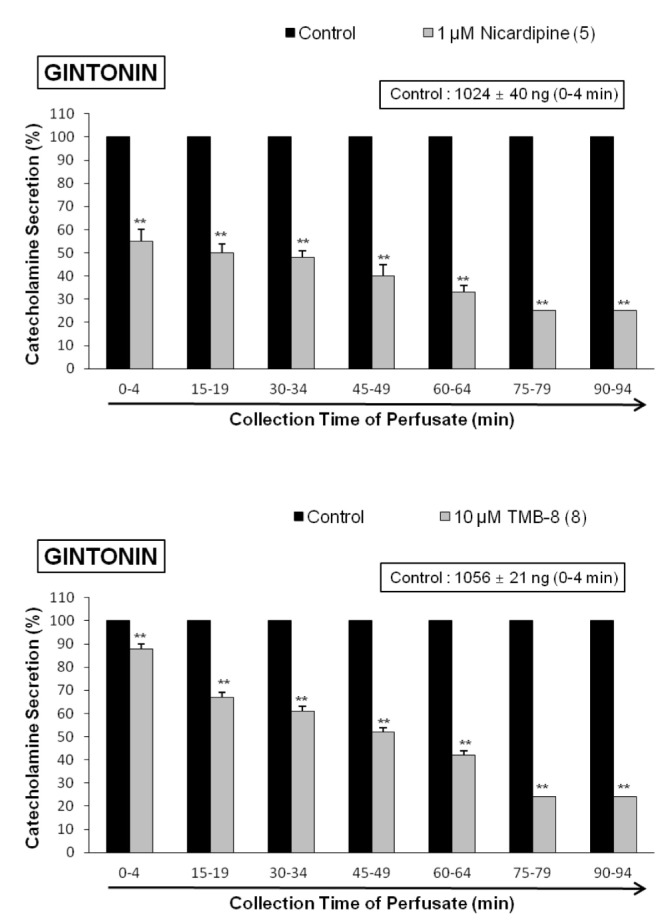
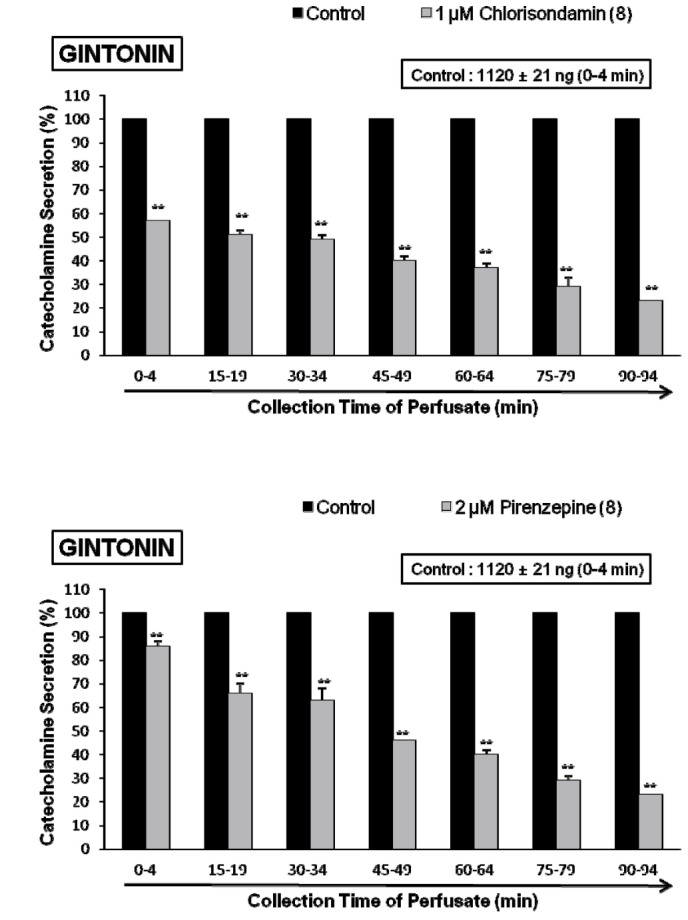
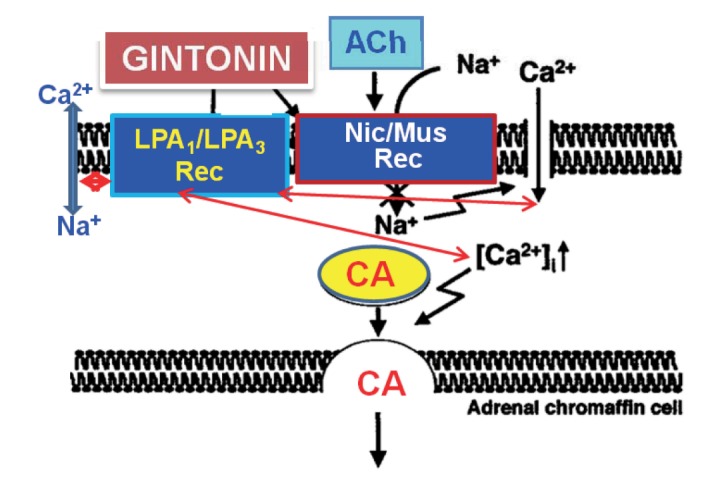
- The present study was designed to investigate the characteristics of gintonin, one of components isolated from Korean Ginseng on secretion of catecholamines (CA) from the isolated perfused model of rat adrenal gland and to clarify its mechanism of action. Gintonin (1 to 30 µg/ml), perfused into an adrenal vein, markedly increased the CA secretion from the perfused rat adrenal medulla in a dose-dependent fashion. The gintonin-evoked CA secretion was greatly inhibited in the presence of chlorisondamine (1 µM, an autonomic ganglionic bloker), pirenzepine (2 µM, a muscarinic Mâ‚ receptor antagonist), Ki14625 (10 µM, an LPAâ‚/₃ receptor antagonist), amiloride (1 mM, an inhibitor of Naâº/Ca²âº exchanger), a nicardipine (1 µM, a voltage-dependent Ca²âº channel blocker), TMB-8 (1 µM, an intracellular Ca²âº antagonist), and perfusion of Ca²âº-free Krebs solution with 5mM EGTA (a Ca²âºchelater), while was not affected by sodium nitroprusside (100 µM, a nitrosovasodialtor). Interestingly, LPA (0.3~3 µM, an LPA receptor agonist) also dose-dependently enhanced the CA secretion from the adrenal medulla, but this facilitatory effect of LPA was greatly inhibited in the presence of Ki 14625 (10 µM). Moreover, acetylcholine (AC)-evoked CA secretion was greatly potentiated during the perfusion of gintonin (3 µg/ml). Taken together, these results demonstrate the first evidence that gintonin increases the CA secretion from the perfused rat adrenal medulla in a dose-dependent fashion. This facilitatory effect of gintonin seems to be associated with activation of LPA- and cholinergic-receptors, which are relevant to the cytoplasmic Ca²âº increase by stimulation of the Ca²âº influx as well as by the inhibition of Ca²âº uptake into the cytoplasmic Ca²âº stores, without the increased nitric oxide (NO). Based on these results, it is thought that gintonin, one of ginseng components, can elevate the CA secretion from adrenal medulla by regulating the Ca²âº mobilization for exocytosis, suggesting facilitation of cardiovascular system. Also, these findings show that gintonin might be at least one of ginseng-induced hypertensive components.
MeSH Terms
-
Acetylcholine
Adrenal Glands
Adrenal Medulla*
Amiloride
Animals
Cardiovascular System
Catecholamines
Chlorisondamine
Cytoplasm
Egtazic Acid
Exocytosis
Ganglia, Autonomic
Nicardipine
Nitric Oxide
Nitroprusside
Panax
Perfusion
Pirenzepine
Rats
Veins
Acetylcholine
Amiloride
Catecholamines
Chlorisondamine
Egtazic Acid
Nicardipine
Nitric Oxide
Nitroprusside
Pirenzepine
Figure
Reference
-
1. Lim DY, Park KB, Kim KH, lee KS, Moon JK, Kim YH. Influence of total ginseng saponin on secretion of catecholamines in the isolated adrenal gland of rabbits. Korean Biochem J. 1987; 20:230–238.2. Lim DY, Park KB, Kim KH, Choi CH, Bae JW, Kim MW. Studies on secretion of catecholamines evoked by panaxadiol in the isolated rabbit adrenal gland. Korean J Pharmacol. 1988; 24:31–42.3. Lim DY, Choi CH, Kim CD, Kim KH, Kim SB, Lee BJ, Chung MH. Influnce of panaxatriol-type saponin on secretion of catecholamine from isolated perfused rabbit adrenal gland. Arch Pharm Res. 1989; 12:166–175.4. Hong SP, Chi H, Cho SH, Lee YK, Woo SC, Kim IS, Oh SH, Yang WH, Lim DY. Influence of total Ginseng saponin on nicotinic stimulation-induced catecholamine secretion from the perfused rat adrenal gland. J Korean Soc Hypertens. 1999; 5:159–168.5. Jang SJ, Lim HJ, Lim DY. Inhibitory Effects of Total Ginseng Saponin on Catecholamine Secretion from the Perfused Adrenal Medulla of SHRs. J Ginseng Res. 2011; 35:176–190. PMID: 23717060.
Article6. Kudo K, Akasaka Y, Miyate Y, Takahashi E, Tachikawa E, Kashimoto T. Effects of red ginseng fractions on catecholamine secretion from bovine adrenal medullary cells. J Med Pharm Soc Wakan-Yaku. 1992; 9:236–239.7. Tachikawa E, Kudo K, Kashimoto T, Takahashi E. Ginseng saponins reduce acetylcholine-evoked Na+ influx and catecholamine secretion in bovine adrenal chromaffin cells. J Pharmacol Exp Ther. 1995; 273:629–636. PMID: 7752064.8. Kudo K, Tachikawa E, Kashimoto T, Takahashi E. Properties of ginseng saponin inhibition of catecholamine secretion in bovine adrenal chromaffin cells. Eur J Pharmacol. 1998; 341:139–144. PMID: 9543231.
Article9. Tachikawa E, Kudo K, Nunokawa M, Kashimoto T, Takahashi E, Kitagawa S. Characterization of ginseng saponin ginsenoside-Rg(3) inhibition of catecholamine secretion in bovine adrenal chromaffin cells. Biochem Pharmacol. 2001; 62:943–951. PMID: 11543730.10. Kim ND, Kang SY, Schini VB. Ginsenosides evoke endotheliumdependent vascular relaxation in rat aorta. Gen Pharmacol. 1994; 25:1071–1077. PMID: 7875528.
Article11. Han KH, Choe SC, Kim HS, Sohn DW, Nam KY, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW. Effect of red ginseng on blood pressure in patients with essential hypertension and white coat hypertension. Am J Chin Med. 1998; 26:199–209. PMID: 9799972.
Article12. Jeon BH, Kim CS, Kim HS, Park JB, Nam KY, Chang SJ. Effect of Korean red ginseng on blood pressure and nitric oxide production. Acta Pharmacol Sin. 2000; 21:1095–1100. PMID: 11603282.13. Jeon BH, Kim CS, Park KS, Lee JW, Park JB, Kim KJ, Kim SH, Chang SJ, Nam KY. Effect of Korea red ginseng on the blood pressure in conscious hypertensive rats. Gen Pharmacol. 2000; 35:135–141. PMID: 11744235.
Article14. Sung J, Han KH, Zo JH, Park HJ, Kim CH, Oh BH. Effects of red ginseng upon vascular endothelial function in patients with essential hypertension. Am J Chin Med. 2000; 28:205–216. PMID: 10999439.
Article15. Siegel RK. Ginseng abuse syndrome. Problems with the panacea. JAMA. 1979; 241:1614–1615. PMID: 430716.
Article16. Baldwin CA, Anderson LA, Phillipson JA. What pharmacists should know about ginseng. Pharm J. 1986; 237:583–586.17. Miller LG. Herbal medicinals: selected clinical considerations focusing on known or potential drug-herb interactions. Arch Intern Med. 1998; 158:2200–2211. PMID: 9818800.18. Klepser TB, Klepser ME. Unsafe and potentially safe herbal therapies. Am J Health Syst Pharm. 1999; 56:125–138. PMID: 10030529.
Article19. Lim DY, Park KB, Kim KH, Moon JK, Lee KS, Kim YK, Chung YH, Hong SP. Influnce of total ginseng saponin on the blood pressure of the rat. Korean Ciru J. 1987; 17:491–499.20. Sohn ES, Park SC, Huh BY, Lee CK, Rhim HK, Ham JS, Yang CM, Han CS, Park CW, Kim HJ. An animal experiental study on the effect of Ginseng on blood pressure and plasma renin activity in spontaneously hypertensive rat. J Korean Med Assoc. 1979; 22:731–746.21. Sohn ES, Park SC, Huh BY, Lee DH, Rhim HK, Young CM, Han CS, Song BS Kim, SJ , Park CW, Kim HJ. An animal experimental study of the effect of Korean Ginseng on body weight and blood pressure in spontaneously hypertensive rat with oral administration. J Korean Med Assoc. 1980; 23:37–48.22. Seok SE, Park CH, Nam SH, Choi HS, Lee JI, Lee DH, Huh BY, Soh ES. An experimental study on the antihypertensive effects of Korea Ginseng to spontanously rats (SHR) in labile stage of hypertension. J Korean Med Assoc. 1981; 24:509–515.23. Sokabe H, Kishi K, Watanabe TX. Effects of Korean red ginseng powder administered orally on blood pressure in hypertensive rats. Proceeding of the 4th international ginseng symposium. Seoul: Korea Ginseng Research Institute;1984. p. 57.24. Stavro PM, Woo M, Leiter LA, Heim TF, Sievenpiper JL, Vuksan V. Long-term intake of North American ginseng has no effect on 24-hour blood pressure and renal function. Hypertension. 2006; 47:791–796. PMID: 16520410.
Article25. Kim ND, Kim EM, Kang KW, Cho MK, Choi SY, Kim SG. Ginsenoside Rg3 inhibits phenylephrine-induced vascular contraction through induction of nitric oxide synthase. Br J Pharmacol. 2003; 140:661–670. PMID: 14534150.
Article26. Han K, Shin IC, Choi KJ, Yun YP, Hong JT, Oh KW. Korea red ginseng water extract increases nitric oxide concentrations in exhaled breath. Nitric Oxide. 2005; 12:159–162. PMID: 15797844.
Article27. Kim ND, Kang SY, Park JH, Schini-Kerth VB. Ginsenoside Rg3 mediates endothelium-dependent relaxation in response to ginsenosides in rat aorta: role of K+ channels. Eur J Pharmacol. 1999; 367:41–49. PMID: 10082263.28. Kang SY, Schini-Kerth VB, Kim ND. Ginsenosides of the protopanaxatriol group cause endothelium-dependent relaxation in the rat aorta. Life Sci. 1995; 56:1577–1586. PMID: 7723586.
Article29. Hien TT, Kim ND, Pokharel YR, Oh SJ, Lee MY, Kang KW. Ginsenoside Rg3 increases nitric oxide production via increases in phosphorylation and expression of endothelial nitric oxide synthase: essential roles of estrogen receptor-dependent PI3-kinase and AMP-activated protein kinase. Toxicol Appl Pharmacol. 2010; 246:171–183. PMID: 20546771.
Article30. Hwang SH, Shin TJ, Choi SH, Cho HJ, Lee BH, Pyo MK, Lee JH, Kang J, Kim HJ, Park CW, Shin HC, Nah SY. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acidsprotein complexes and activates G protein-coupled lysophosphatidic acid receptors with high affinity. Mol Cells. 2012; 33:151–162. PMID: 22286231.
Article31. Nah SY, Kim DH, Rhim H. Ginsenosides: are any of them candidates for drugs acting on the central nervous system? CNS Drug Rev. 2007; 13:381–404. PMID: 18078425.
Article32. Pyo MK, Choi SH, Hwang SH, Shin TJ, Lee BH, Lee SM, Lim YH, Kim DH, Nah SY. Novel glycoproteins from ginseng. J Ginseng Res. 2011; 35:92–103.33. Hwang SH, Lee BH, Choi SH, Kim HJ, Jung SW, Kim HS, Shin HC, Park HJ, Park KH, Lee MK, Nah SY. Gintonin, a novel ginsengderived lysophosphatidic acid receptor ligand, stimulates neurotransmitter release. Neurosci Lett. 2015; 584:356–361. PMID: 25445364.
Article34. Wakade AR. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J Physiol. 1981; 313:463–480. PMID: 7277230.
Article35. Anton AH, Sayre DF. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962; 138:360–375. PMID: 14013351.36. Tallarida RJ, Murray RB. Manual of pharmacologic calculation with computer programs. 2nd ed. New York: Speringer-Verlag;1987. p. 132.37. Tachikawa E, Kudo K, Hasegawa H, Kashimoto T, Sasaki K, Miyazaki M, Taira H, Lindstrom JM. In vitro inhibition of adrenal catecholamine secretion by steroidal metabolites of ginseng saponins. Biochem Pharmacol. 2003; 66:2213–2221. PMID: 14609746.
Article38. Dixon WR, Garcia AG, Kirpekar SM. Release of catecholamines and dopamine beta-hydroxylase from the perfused adrenal gland of the cat. J Physiol. 1975; 244:805–824. PMID: 1133780.
Article39. Hardman JG, Limbird LE, Molinoff PB, Ruddon RR, Gilman AG. Goodman & Gilman's the pharmacological basis of therapeutics. 9th ed. New York: McGrraw-Hill;1995. p. 193–195.40. Nakazato Y, Ohga A, Oleshansky M, Tomita U, Yamada Y. Voltage-independent catecholamine release mediated by the activation of muscarinic receptors in guinea-pig adrenal glands. Br J Pharmacol. 1988; 93:101–109. PMID: 3349226.
Article41. Lim DY, Kim CD, Ahn GW. Influence of TMB-8 on secretion of catecholamines from the perfused rat adrenal glands. Arch Pharm Res. 1992; 15:115–125.
Article42. Yu L, Netticadan T, Xu YJ, Panagia V, Dhalla NS. Mechanisms of lysophosphatidylcholine-induced increase in intracellular calcium in rat cardiomyocytes. J Pharmacol Exp Ther. 1998; 286:1–8. PMID: 9655835.43. Rathi SS, Saini HK, Xu YJ, Dhalla NS. Mechanisms of low Na+-induced increase in intracellular calcium in KCl-depolarized rat cardiomyocytes. Mol Cell Biochem. 2004; 263:151–162.44. Ohta H, Sato K, Murata N, Damirin A, Malchinkhuu E, Kon J, Kimura T, Tobo M, Yamazaki Y, Watanabe T, Yagi M, Sato M, Suzuki R, Murooka H, Sakai T, Nishitoba T, Im DS, Nochi H, Tamoto K, Tomura H, Okajima F. Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol Pharmacol. 2003; 64:994–1005. PMID: 14500756.
Article45. Shiono S, Kawamoto K, Yoshida N, Kondo T, Inagami T. Neurotransmitter release from lysophosphatidic acid stimulated PC12 cells: involvement of lysophosphatidic acid receptors. Biochem Biophys Res Commun. 1993; 193:667–673. PMID: 8390248.46. Pan CY, Lee H, Chen CL. Lysophospholipids elevate [Ca2+]i and trigger exocytosis in bovine chromaffin cells. Neuropharmacology. 2006; 51:18–26. PMID: 16616768.47. Viveros OH. Mechanism of secretion of catecholaminies from adrenal medulla. In : Blaschko H, Sayers G, Smith DA, editors. Handbook of physiology, Endocrinology. Vol VI, Sect 7, The adrenal gland. Washington DC: American physiological society;1975. p. 389–426. .48. Viveros OH, Arqueros LC, Kirshner N. Release of catecholanines and dopamine beta-hydroxylase from the adrenal medulla. Lifr Sci. 1968; 7:609–618.49. Eglen RM, Whiting RL. Muscarinic receptor subtypes: a critique of the current classification and a proposal for a working nomenclature. J Auton Pharmacol. 1986; 6:323–346. PMID: 3546321.
Article50. Hammer R, Berrie CP, Birdsall NJ, Burgen AS, Hulme EC. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature. 1980; 283:90–92. PMID: 7350532.
Article51. Hammer R, Giachetti A. Muscarinic receptor subtypes: M1 and M2 biochemical and functional characterization. Life Sci. 1982; 31:2991–2998. PMID: 6897666.52. Doods HN, Mathy MJ, Davidesko D, van Charldorp KJ, de Jonge A, van Zwieten PA. Selectivity of muscarinic antagonists in radioligand and in vivo experiments for the putative M1, M2 and M3 receptors. J Pharmacol Exp Ther. 1987; 242:257–262. PMID: 3612532.53. Liu JX, Cong WH, Xu L, Wang JN. Effect of combination of extracts of ginseng and ginkgo biloba on acetylcholine in amyloid betaprotein-treated rats determined by an improved HPLC. Acta Pharmacol Sin. 2004; 25:1118–1123. PMID: 15339385.54. Su CF, Cheng JT, Liu IM. Increase of acetylcholine release by Panax ginseng root enhances insulin secretion in Wistar rats. Neurosci Lett. 2007; 412:101–104. PMID: 17123721.
Article55. Kim H, Lee BH, Choi SH, Kim HJ1, Jung SW, Hwang SH, Rhim H, Kim HC, Cho IH, Nah SY. Gintonin stimulates gliotransmitter release in cortical primary astrocytes. Neurosci Lett. 2015; 603:19–24. PMID: 26191656.
Article56. Douglas WW. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968; 34:451–474. PMID: 4882190.
Article57. Schulz I, Stolze HH. The exocrine pancreas: the role of secretagogues, cyclic nucleotides, and calcium in enzyme secretion. Annu Rev Physiol. 1980; 42:127–156. PMID: 6105844.
Article58. Williams JA. Regulation of pancreatic acinar cell function by intracellular calcium. Am J Physiol. 1980; 238:G269–G279. PMID: 6155077.
Article59. Mizobe F, Livett BG. Nicotine stimulates secretion of both catecholamines and acetylcholinesterase from cultured adrenal chromaffin cells. J Neurosci. 1983; 3:871–876. PMID: 6834109.
Article60. Wakade AR, Wakade TD. Contribution of nicotinic and muscarinic receptors in the secretion of catecholamines evoked by endogenous and exogenous acetylcholine. Neuroscience. 1983; 10:973–978. PMID: 6139771.
Article61. Kilpatrick DL, Slepetis R, Kirshner N. Ion channels and membrane potential in stimulus-secretion coupling in adrenal medulla cells. J Neurochem. 1981; 36:1245–1255. PMID: 6259284.
Article62. Kilpatrick DL, Slepetis RJ, Corcoran JJ, Kirshner N. Calcium uptake and catecholamine secretion by cultured bovine adrenal medulla cells. J Neurochem. 1982; 38:427–435. PMID: 7108549.
Article63. Knight DE, Kesteven NT. Evoked transient intracellular free Ca2+ changes and secretion in isolated bovine adrenal medullary cells. Proc R Soc Lond B Biol Sci. 1983; 218:177–199. PMID: 6135214.64. Misbahuddin M, Isosaki M, Houchi H, Oka M. Muscarinic receptor-mediated increase in cytoplasmic free Ca2+ in isolated bovine adrenal medullary cells. Effects of TMB-8 and phorbol ester TPA. FEBS Lett. 1985; 190:25–28. PMID: 4043396.65. Sasakawa N, Yamamoto S, Ishii K, Kato R. Inhibition of calcium uptake and catecholamine release by 8-(N,N-diethylamino)-octyl-3,4,5-trimethoxybenzoate hydrochloride (TMB-8) in cultured bovine adrenal chromaffin cells. Biochem Pharmacol. 1984; 33:4063–4067. PMID: 6508853.66. Yamada Y, Teraoka H, Nakazato Y, Ohga A. Intracellular Ca2+ antagonist TMB-8 blocks catecholamine secretion evoked by caffeine and acetylcholine from perfused cat adrenal glands in the absence of extracellular Ca2+. Neurosci Lett. 1988; 90:338–342. PMID: 3419642.67. Lim DY, Lee JH, Kim WS, Kim SB, Lee EH, Lee BJ, Ko ST. Studies on secretion of catecholamine evoked by caffeine from the isolated perfused rat adrenal gland. Arch Pharm Res. 1991; 14:55–67. PMID: 10319123.
Article68. Wang B, Zhang XQ, Liu TP, Xiao JG. 8-(N,N-diethylamino)-n-octyl-3,4,5-trimethoxybenzoate reduced [Ca2+]i elevation induced by histamine, serotonin, and glutamate in cultured calf basilar artery smooth muscle cells. Zhongguo Yao Li Xue Bao. 1998; 19:251–253. PMID: 10375737.69. Zhang XQ, Wang B, Zhang MY, Xiao JG. Inhibitory effects of 8-(N,N-diethylamino)-n-octyl-3,4,5-trimethoxybenzoate (TMB-8) on intracellular Ca2+ elevated by neurotransmitters in brain cells. Zhongguo Yao Li Xue Bao. 1999; 20:893–896. PMID: 11270987.70. Zhang XQ, Wang B, Zhang MY, Xiao JG. [Effect of TMB-8 on the increase of intracellular free Ca2+ induced by NE and BHQ in dissociated single rat brain cell]. Yao Xue Xue Bao. 1997; 32:726–730. PMID: 11596212.71. Benos DJ. Amiloride: a molecular probe of sodium transport in tissues and cells. Am J Physiol. 1982; 242:C131–C145. PMID: 7039345.
Article72. Schellenberg GD, Anderson L, Swanson PD. Inhibition of Na+-Ca2+ exchange in rat brain by amiloride. Mol Pharmacol. 1983; 24:251–258. PMID: 6888368.73. Siegl PK, Cragoe EJ Jr, Trumble MJ, Kaczorowski GJ. Inhibition of Na+/Ca2+ exchange in membrane vesicle and papillary muscle preparations from guinea pig heart by analogs of amiloride. Proc Natl Acad Sci U S A. 1984; 81:3238–3242. PMID: 6587348.74. Houchi H, Okuno M, Tokumura A, Yoshizumi M, Fukuzawa K, Oka M. Lysophosphatidic acid as a stimulator of Na+-dependent Ca2+ efflux from adrenal chromaffin cells. Life Sci. 1995; 57:PL205–PL210. PMID: 7674825.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Corrigendum to: Gintonin facilitates catecholamine secretion from the perfused adrenal medulla
- Inhibitory Effects of Olmesartan on Catecholamine Secretion from the Perfused Rat Adrenal Medulla
- Influence of SKF81297 on Catecholamine Release from the Perfused Rat Adrenal Medulla
- Mechanism of Leptin-Induced Potentiation of Catecholamine Secretion Evoked by Cholinergic Stimulation in the Rat Adrenal Medulla
- Suppressive Impact of Ginsenoside-Rg2 on Catecholamine Secretion from the Rat Adrenal Medulla