J Korean Med Assoc.
2015 May;58(5):433-440. 10.5124/jkma.2015.58.5.433.
Present and future of allergen immunotherapy for allergic diseases
- Affiliations
-
- 1Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, Korea. dhnahm@gmail.com
- KMID: 2195039
- DOI: http://doi.org/10.5124/jkma.2015.58.5.433
Abstract
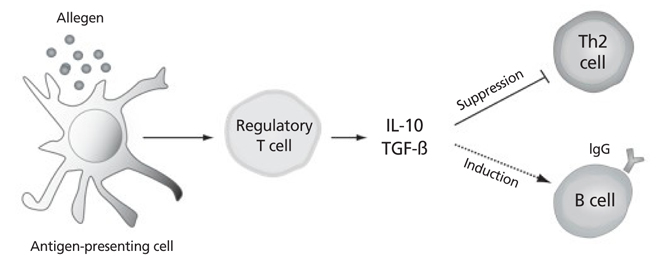
- Current pharmacological therapies for allergic diseases can improve clinical symptoms but cannot change their long-term clinical course. There is an unmet need for a curative treatment for allergic diseases. Allergen immunotherapy (AIT) is the practice of administering increasing doses of clinically relevant allergens to an allergic subject to reduce the clinical symptoms associated with subsequent exposure to the allergen. AIT is clinically effective for allergic asthma, allergic rhinitis, venom-induced anaphylaxis, and atopic dermatitis. AIT can change the natural course of allergic diseases and induce allergen-specific immune tolerance. In current clinical practice, AIT is delivered either subcutaneously or sublingually. Both subcutaneous and sublingual AIT have long-term therapeutic efficacy after of 3-5 years of treatment. The development of safer and more effective AIT strategies is needed. Conclusion: AIT is a disease-modifying therapy for allergic diseases. Future development of AIT should be directed toward achieving long-term clinical remission in patients with allergic diseases by the safe and effective induction of immune tolerance.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Safety of ultrarush allergen subcutaneous immunotherapy in children with allergic disease
Sang Won Cho, Gun Moo Lee, Jin Sung Park, Jae Woo Kwon, Ja Kyoung Kim
Allergy Asthma Respir Dis. 2017;5(6):336-343. doi: 10.4168/aard.2017.5.6.336.
Reference
-
1. Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, Williams H. ISAAC Phase Three Study Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet. 2006; 368:733–743.
Article2. Akdis CA. Therapies for allergic inflammation: refining strategies to induce tolerance. Nat Med. 2012; 18:736–749.
Article3. Global Initiative for Asthma. 2012 Update: global strategy for asthma management and prevention [Internet]. [place unknown]: Global Initiative for Asthma;2012. 2015 Apr 21. Available from: http://www.ginasthma.org/documents/5/documents_variants/37.4. Park JW. Allergen specific immunotherapy for allergic rhinitis. Korean J Med. 2013; 84:798–801.
Article5. Akdis CA, Akdis M, Bieber T, Bindslev-Jensen C, Boguniewicz M, Eigenmann P, Hamid Q, Kapp A, Leung DY, Lipozencic J, Luger TA, Muraro A, Novak N, Platts-Mills TA, Rosenwasser L, Scheynius A, Simons FE, Spergel J, Turjanmaa K, Wahn U, Weidinger S, Werfel T, Zuberbier T. European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma and Immunology. Diagnosis and treatment of atopic dermatitis in children and adults: European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma and Immunology/PRACTALL Consensus Report. J Allergy Clin Immunol. 2006; 118:152–169.
Article6. Schafer T. Epidemiology of complementary alternative medicine for asthma and allergy in Europe and Germany. Ann Allergy Asthma Immunol. 2004; 93:2 Suppl 1. S5–S10.7. Jung JH, Lee JH, Kang IG, Cha HE, Kim ST. The present state of Korean traditional medicine and alternative medicine in nasal disease. Korean J Otorhinolaryngol-Head Neck Surg. 2010; 53:12–19.
Article8. Waldmann TA. Immunotherapy: past, present and future. Nat Med. 2003; 9:269–277.
Article9. Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012; 366:2517–2519.
Article10. Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases: a WHO position paper. J Allergy Clin Immunol. 1998; 102(4 Pt 1):558–562.
Article11. Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, Nelson H, Akdis CA. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013; 131:1288–1296.
Article12. Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, Nelson M, Weber R, Bernstein DI, Blessing-Moore J, Khan DA, Lang DM, Nicklas RA, Oppenheimer J, Portnoy JM, Randolph C, Schuller DE, Spector SL, Tilles S, Wallace D. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011; 127:1 Suppl. S1–S55.13. Hur GY, Kim TB, Kim ST, Han MY, Nahm DH, Lee YW, Sohn SW, Lee HH, Kim WK, Song TW, Kim S, Kim SH, Park JW. Allergy immunotherapy. Korean J Asthma Allergy Clin Immunol. 2010; 30:153–183.14. Noon L. Prophylactic inoculation against hay fever. Lancet. 1911; 177:1572–1573.
Article15. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010; (8):CD001186.
Article16. Calderon MA, Alves B, Jacobson M, Hurwitz B, Sheikh A, Durham S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007; (1):CD001936.
Article17. Boyle RJ, Elremeli M, Hockenhull J, Cherry MG, Bulsara MK, Daniels M, Oude Elberink JN. Venom immunotherapy for preventing allergic reactions to insect stings. Cochrane Database Syst Rev. 2012; 10:CD008838.
Article18. Bae JM, Choi YY, Park CO, Chung KY, Lee KH. Efficacy of allergen-specific immunotherapy for atopic dermatitis: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2013; 132:110–117.
Article19. Pipet A, Botturi K, Pinot D, Vervloet D, Magnan A. Allergenspecific immunotherapy in allergic rhinitis and asthma: mechanisms and proof of efficacy. Respir Med. 2009; 103:800–812.
Article20. Radulovic S, Calderon MA, Wilson D, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2010; (12):CD002893.
Article21. Calderon MA, Penagos M, Sheikh A, Canonica GW, Durham S. Sublingual immunotherapy for treating allergic conjunctivitis. Cochrane Database Syst Rev. 2011; (7):CD007685.
Article22. Shakir EM, Cheung DS, Grayson MH. Mechanisms of immunotherapy: a historical perspective. Ann Allergy Asthma Immunol. 2010; 105:340–347.
Article23. Jutel M, Van de Veen W, Agache I, Azkur KA, Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy and novel ways for vaccine development. Allergol Int. 2013; 62:425–433.
Article24. Passalacqua G, Compalati E, Canonica GW. Advances in allergen-specific immunotherapy. Curr Drug Targets. 2009; 10:1255–1262.
Article25. Casale TB, Stokes JR. Future forms of immunotherapy. J Allergy Clin Immunol. 2011; 127:8–15.
Article26. Pauli G, Larsen TH, Rak S, Horak F, Pastorello E, Valenta R, Purohit A, Arvidsson M, Kavina A, Schroeder JW, Mothes N, Spitzauer S, Montagut A, Galvain S, Melac M, Andre C, Poulsen LK, Malling HJ. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008; 122:951–960.
Article27. Holgate ST, Djukanovic R, Casale T, Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy. 2005; 35:408–416.
Article28. Beck LA, Thaci D, Hamilton JD, Graham NM, Bieber T, Rocklin R, Ming JE, Ren H, Kao R, Simpson E, Ardeleanu M, Weinstein SP, Pirozzi G, Guttman-Yassky E, Suarez-Farinas M, Hager MD, Stahl N, Yancopoulos GD, Radin AR. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014; 371:130–139.
Article29. Vatrella A, Fabozzi I, Calabrese C, Maselli R, Pelaia G. Dupilumab: a novel treatment for asthma. J Asthma Allergy. 2014; 7:123–130.
Article30. Nahm DH, Lee ES, Park HJ, Kim HA, Choi GS, Jeon SY. Treatment of atopic dermatitis with a combination of allergen-specific immunotherapy and a histamine-immunoglobulin complex. Int Arch Allergy Immunol. 2008; 146:235–240.
Article