J Korean Neuropsychiatr Assoc.
2016 Feb;55(1):25-32. 10.4306/jknpa.2016.55.1.25.
The Correlation between Clinical Symptoms, Serum Uric Acid Level and EEG in Patient with Bipolar I Disorder
- Affiliations
-
- 1Department of Psychiatry, Chung-Ang University College of Medicine, Seoul, Korea. sunmikim706@gmail.com
- KMID: 2192905
- DOI: http://doi.org/10.4306/jknpa.2016.55.1.25
Abstract
OBJECTIVES
High uric acid level is related to increased locomotor activities and refractory mood swings. The purpose of this study is to examine the correlation between clinical symptoms of mania, serum uric acid level, and quantitative electroencephalography (QEEG) findings.
METHODS
Twenty-four patients with bipolar disorder and 24 healthy control subjects agreed to participate in the study. When they were hospitalized, the degree of clinical symptoms, uric acid level in blood, and brain QEEG were measured.
RESULTS
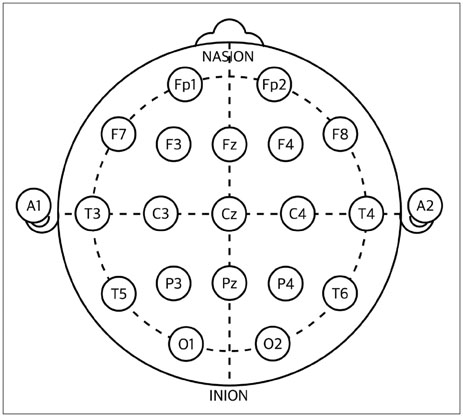
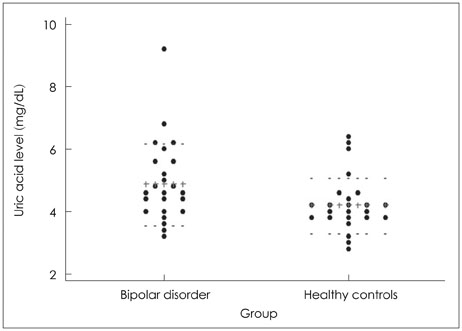
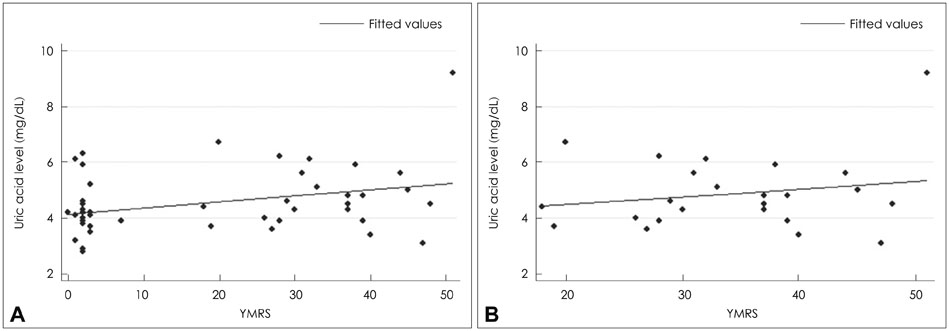
The bipolar disorder group showed higher scores on the Young Mania Rating Scale (YMRS ; z=6.02, p<0.05). Patients in their manic episodes showed higher plasma uric acid levels (4.9+/-1.3 mg/dL) than healthy control subjects (4.2+/-0.9 mg/dL ; z=2.14, p<0.05). Uric acid levels showed correlation with severity of manic symptoms as assessed using the YMRS in all participants (rho=0.28, p<0.05). The bipolar disorder group showed decreased relative delta and alpha activity in the fronto-temporo-occipital region compared to the control group (p<0.05). Relative beta in Fp1 (frontopolar), Cz (central mid-line), and Pz (parietal mid-line) and relative gamma in Fp1 were increased in the bipolar disorder group, relative to the control group (p<0.05). The relative beta (rho=0.47, p<0.05) and gamma (rho=0.41, p<0.05) in Fp1 electrodes showed positive correlation with the YMRS scores.
CONCLUSION
Adenosinergic transmission dysfunction may lead to occurrence of manic symptoms, considering that a key role of central nervous system adenosinergic receptors is to inhibit the release of various neurotransmitters and limit neuronal excitability. In addition, QEEG appeared to indicate excitatory neuro-modulation in manic patients.
MeSH Terms
Figure
Reference
-
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). Arlington, VA: American Psychiatric Association;2013.2. Mitchell PB, Hadzi-Pavlovic D. Lithium treatment for bipolar disorder. Bull World Health Organ. 2000; 78:515–517.3. Machado-Vieira R, Lara DR, Souza DO, Kapczinski F. Purinergic dysfunction in mania: an integrative model. Med Hypotheses. 2002; 58:297–304.
Article4. Salvadore G, Viale CI, Luckenbaugh DA, Zanatto VC, Portela LV, Souza DO, et al. Increased uric acid levels in drug-naïve subjects with bipolar disorder during a first manic episode. Prog Neuropsychopharmacol Biol Psychiatry. 2010; 34:819–821.
Article5. Simonato M, Varani K, Muzzolini A, Bianchi C, Beani L, Borea PA. Adenosine A1 receptors in the rat brain in the kindling model of epilepsy. Eur J Pharmacol. 1994; 265:121–124.
Article6. Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol. 2005; 63:191–270.
Article7. Waldeck B. Effect of caffeine on locomotor activity and central catecholamine mechanisms: a study with special reference to drug interaction. Acta Pharmacol Toxicol (Copenh). 1975; 36:Suppl 4. 1–23.
Article8. Holmes EW, Kelley WN, Wyngaarden JB. Control of purine biosynthesis in normal and pathologic states. Bull Rheum Dis. 1975-1976; 26:848–853.9. Machado-Vieira R, Lara DR, Souza DO, Kapczinski F. Therapeutic efficacy of allopurinol in mania associated with hyperuricemia. J Clin Psychopharmacol. 2001; 21:621–622.
Article10. Machado-Vieira R, Soares JC, Lara DR, Luckenbaugh DA, Busnello JV, Marca G, et al. A double-blind, randomized, placebo-controlled 4-week study on the efficacy and safety of the purinergic agents allopurinol and dipyridamole adjunctive to lithium in acute bipolar mania. J Clin Psychiatry. 2008; 69:1237–1245.
Article11. Anumonye A, Reading HW, Knight F, Ashcroft GW. Uric-acid metabolism in manic-depressive illness and during lithium therapy. Lancet. 1968; 1:1290–1293.
Article12. Painold A, Faber PL, Milz P, Reininghaus EZ, Holl AK, Letmaier M, et al. Brain electrical source imaging in manic and depressive episodes of bipolar disorder. Bipolar Disord. 2014; 16:690–702.
Article13. Clementz BA, Sponheim SR, Iacono WG, Beiser M. Resting EEG in first-episode schizophrenia patients, bipolar psychosis patients, and their first-degree relatives. Psychophysiology. 1994; 31:486–494.
Article14. Small JG, Milstein V, Malloy FW, Klapper MH, Golay SJ, Medlock CE. Topographic EEG studies of mania. Clin Electroencephalogr. 1998; 29:59–66.
Article15. American Electroencephalographic Society guidelines for standard electrode position nomenclature. J Clin Neurophysiol. 1991; 8:200–202.16. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978; 133:429–435.
Article17. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960; 23:56–62.
Article18. Hamilton M. Rating depressive patients. J Clin Psychiatry. 1980; 41(12 Pt 2):21–24.19. Yi JS, Bae SO, Ahn YM, Park DB, Noh KS, Shin HK, et al. Validity and reliability of the Korean version of the Hamilton Depression Rating Scale(K-HDRS). J Korean Neuropsychiatr Assoc. 2005; 44:456–465.20. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961; 4:561–571.
Article21. Hahn HM, Yum TH, Shin YW, Kim KH, Yoon DJ, Chung KJ. A standardization study of Beck Depression Inventory in Korea. J Korean Neuropsychiatr Assoc. 1986; 25:487–502.22. Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988; 56:893–897.
Article23. Yook SP, Kim JS. A clinical study on the Korean version of Beck Anxiety Inventory: comparative study of patient and non-patient. Korean J Clin Psychol. 1997; 16:185–197.24. Albert U, De Cori D, Aguglia A, Barbaro F, Bogetto F, Maina G. Increased uric acid levels in bipolar disorder subjects during different phases of illness. J Affect Disord. 2015; 173:170–175.
Article25. De Berardis D, Conti CM, Campanella D, Carano A, Di Giuseppe B, Valchera A, et al. Evaluation of plasma antioxidant levels during different phases of illness in adult patients with bipolar disorder. J Biol Regul Homeost Agents. 2008; 22:195–200.26. Kesebir S, Tatlıdil Yaylacı E, Süner O, Gültekin BK. Uric acid levels may be a biological marker for the differentiation of unipolar and bipolar disorder: the role of affective temperament. J Affect Disord. 2014; 165:131–134.
Article27. Gast H, Schindler K, Rummel C, Herrmann US, Roth C, Hess CW, et al. EEG correlation and power during maintenance of wakefulness test after sleep-deprivation. Clin Neurophysiol. 2011; 122:2025–2031.
Article28. Mitra S, Nizamie SH, Goyal N, Tikka SK. Evaluation of resting state gamma power as a response marker in schizophrenia. Psychiatry Clin Neurosci. 2015; 69:630–639.
Article29. Davidson RJ. Affective style and affective disorders: perspectives from affective neuroscience. Cogn Emot. 1998; 12:307–330.
Article30. Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. J Abnorm Psychol. 1991; 100:535–545.
Article31. Hammond DC. Neurofeedback with anxiety and affective disorders. Child Adolesc Psychiatr Clin N Am. 2005; 14:105–123. vii
Article32. Degabriele R, Lagopoulos J. A review of EEG and ERP studies in bipolar disorder. Acta Neuropsychiatr. 2009; 21:58–66.
Article33. Strakowski SM, DelBello MP, Adler C, Cecil DM, Sax KW. Neuroimaging in bipolar disorder. Bipolar Disord. 2000; 2(3 Pt 1):148–164.
Article34. Blumberg HP, Kaufman J, Martin A, Charney DS, Krystal JH, Peterson BS. Significance of adolescent neurodevelopment for the neural circuitry of bipolar disorder. Ann N Y Acad Sci. 2004; 1021:376–383.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Observation of the Serum Uric Acid in Essential Hypertension
- Study on Serum and Urinary Uric Acid Level in Patients with Urinary Stone
- Changes of Symptoms and Serum Lithium Levels in Patient with Bipolar Disorder According to Menstrual Cycle
- Serum Uric Acid Levels In Korean Adult Population And Their Correlates
- Interference of Bilirubin in Measurement of Uric Acid